Question
Question: Resonance hybrid of nitrate ion is ………. A. 
B. 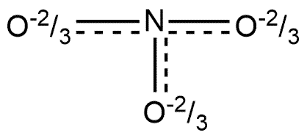
C. 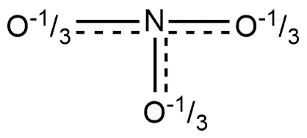
D. 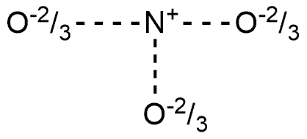
Solution
In chemistry resonance can be defined as a way of describing bonding in certain molecules or ions by the combination of different contributing structures and the structures formed during the process of resonance are known as resonating or canonical structures.
Complete step by step answer:
- During the process of resonance resonating structure turned into a resonance hybrid also known as hybrid structure. It contains a particular value which generally describes delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis structure.
Lewis structure of nitrate ion can be shown by:
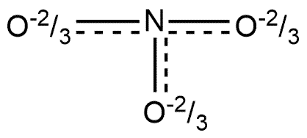
- According to resonance theory each bond in the nitrate ion is one and one third of a bond present between nitrogen and oxygen which is consistent with the observation that the three bonds in the nitrate ion have the same bond length and the same bond energy. It can be explained as we know that oxygen carries -1 charge in this case and nitrogen carries neutral charge so average charge of O−N−O is given by
1→−12→03→−1
Here 1 and 3 refers with O and 2 refers with N. Weighted average will be given by:
(−1×31)+(0×31)+(−1×31)=3−2
In all three cases the weighted average will remain the same i.e. 3−2.
The correct answer is option “B” .
Note: Hybrid is a compound, molecule, ion or radical exhibiting the property of resonance and having a structure which can be represented in the written form as the average of two or more structural formulas separated each from the next by a double-headed arrow.
