Question
Question: Representing \(P,V\) and \(T\) as pressure, volume and temperature respectively ,which of the follow...
Representing P,V and T as pressure, volume and temperature respectively ,which of the following is the correct representation of Boyle’s Law?
A. V∝T1 (P constant )
B. V∝P1 (T constant )
C. PV=RT
D. PV=nRT
Solution
Hint: We have studied that Boyle’s Law is concerning the compression and expansion of a gas at constant temperature. Robert Boyle’s states that the pressure of a quantity of gas varies inversely with its volume at constant temperature.
Complete answer:
This law can be derived from the kinetic theory of gases. It is a law for ideal gases (Ideal gas helps in prediction of real gases). In simple terms we can say that the volume of an ideal gas at constant temperature is inversely proportional to the pressure applied on it. The relationship between pressure and volume at constant temperature is expressed as follows,
⇒P∝V1, where P is the pressure and V is the volume of the gas. Now to remove the proportionality sign we will convert it into an equation by adding a constant.
⇒P=k×(V1)
⇒PV=k= constant
The above equation can also be shown in graph ,the relation between pressure and volume can be shown in graph,
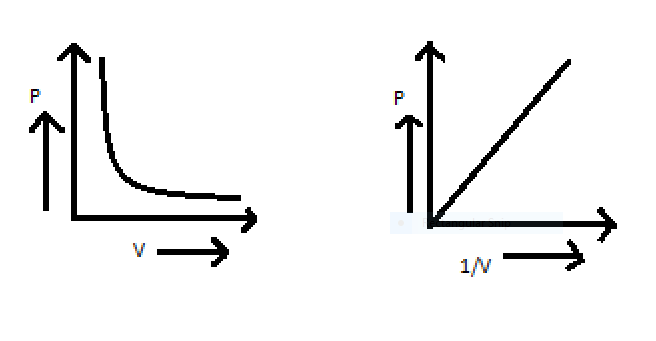 To get the answer of the problem we will take help of ideal gas equation that is PV=nRT
To get the answer of the problem we will take help of ideal gas equation that is PV=nRT
⇒PV= constant =k
⇒V=Pk
⇒V∝p1 so option B that is V∝P1 (T constant) is the correct representation of Boyle’s Law.
Note: Now we know that in Boyle’s Law ,’’ Any change in the volume of the gas at constant temperature will result in change in the pressure applied by it”. Mathematically this law is expressed as P1V1=P2V2 where P1,P2,V1,V2 are respectively initial pressure, final pressure , initial volume, final volume. We should remember that this law holds true only when the number of moles and temperature both are constant.
