Question
Question: Represent each of the following atoms using Lewis notation: (a) Beryllium (b) Calcium (c) Lith...
Represent each of the following atoms using Lewis notation:
(a) Beryllium
(b) Calcium
(c) Lithium
Solution
To determine the answer we should know what Lewis notation is. Lewis notation is a way to show the valence shell electrons of an atom or the bonded and non-bonded electron pairs of the molecules. Lewis notation represents the electron as a dot around the symbol of the atoms.
Complete solution:
We will use the following rules to write the Lewis structure:
For an atom:
We will write the electronic configuration of the atom to determine the valence electrons.
Then we will write the symbol of the atom.
Then we will represent each valence electron as a dot around the symbol of the atom.
(a) Beryllium
The electronic configuration of the beryllium is,1s22s2.
Valence electrons of the beryllium are 2.
The symbol of beryllium is Be.
So, the Lewis notation of beryllium is,
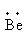
(b) Calcium
The electronic configuration of the calcium is,1s22s22p63s23p64s2.
Valence electrons of the calcium are 2.
The symbol of calcium is Ca.
So, the Lewis notation of calcium is,
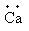
(c) Lithium
The electronic configuration of the lithium is,1s22s1.
Valence electrons of the lithium are 1.
The symbol of lithium is Li.
So, the Lewis notation of lithium is,
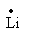
Addition information: The rules to write the Lewis notation for molecules is as follows:
We write the basic structure. Then decide the central atom around which we write all atoms of the molecule. The least electronegative atom is the central atom.
-Then we count the total of valence electrons.
-Two electrons are used in the formation of a bond.
-Then we count the total electron used in bond formation.
-We subtract the electrons used in bond formation from the total valence electrons.
Note: The electrons present in the outermost shell are known as valence shell electrons. We show only valence shell electrons in Lewis notation, not the inner shell electrons. According to octet rule, any atom requires eight electrons to complete an octet but hydrogen requires two electrons to complete its octet. The lone pair electron density lies only on one atom whereas the bond pair electron density lies in between two bonded atoms.
