Question
Question: Relative charge on electron is : A. -1 B. +1 C. 0 D. -2...
Relative charge on electron is :
A. -1
B. +1
C. 0
D. -2
Solution
J.J. Thomson studied cathode rays and discovered electrons. Electrons are equal but opposite to the charge of a proton. All atoms contain the same number of electrons as protons, so the positive and negative charges call off making atoms electrically neutral.
Complete step by step answer:
Atoms are mainly composed of three subatomic particles namely protons, electrons and neutrons.
Electrons: The negatively charged particles that revolve around the nucleus of an atom are called electrons. They have a mass of 9.1093×10−31kg. They are represented as “e”. The charge on an electron is −1. They participate in both chemical and nuclear reactions.
Protons: Protons are positively charged particles present inside the nucleus. A proton is represented as “p”. The mass of a proton is 1.672×10−27kg. Protons take part in the nuclear reactions. The charge of a proton is +1.
Neutrons: The neutral particles (no charge) that are present inside the nucleus of an atom are called neutrons. The mass of neutrons is slightly higher than the protons. They are represented as “n” and they are exposed to nuclear reactions. The charge of a neutron is zero.
The combination of the number of protons and number of neutrons present inside a nucleus of an atom is called a nucleon.
So, the correct answer is Option A.
Note:
Mass number is the sum of the protons and neutrons in the nucleus.
Atomic number is the number of protons present in the nucleus.
For an element E, the top left number represents the mass number and the bottom left number represents the atomic number.
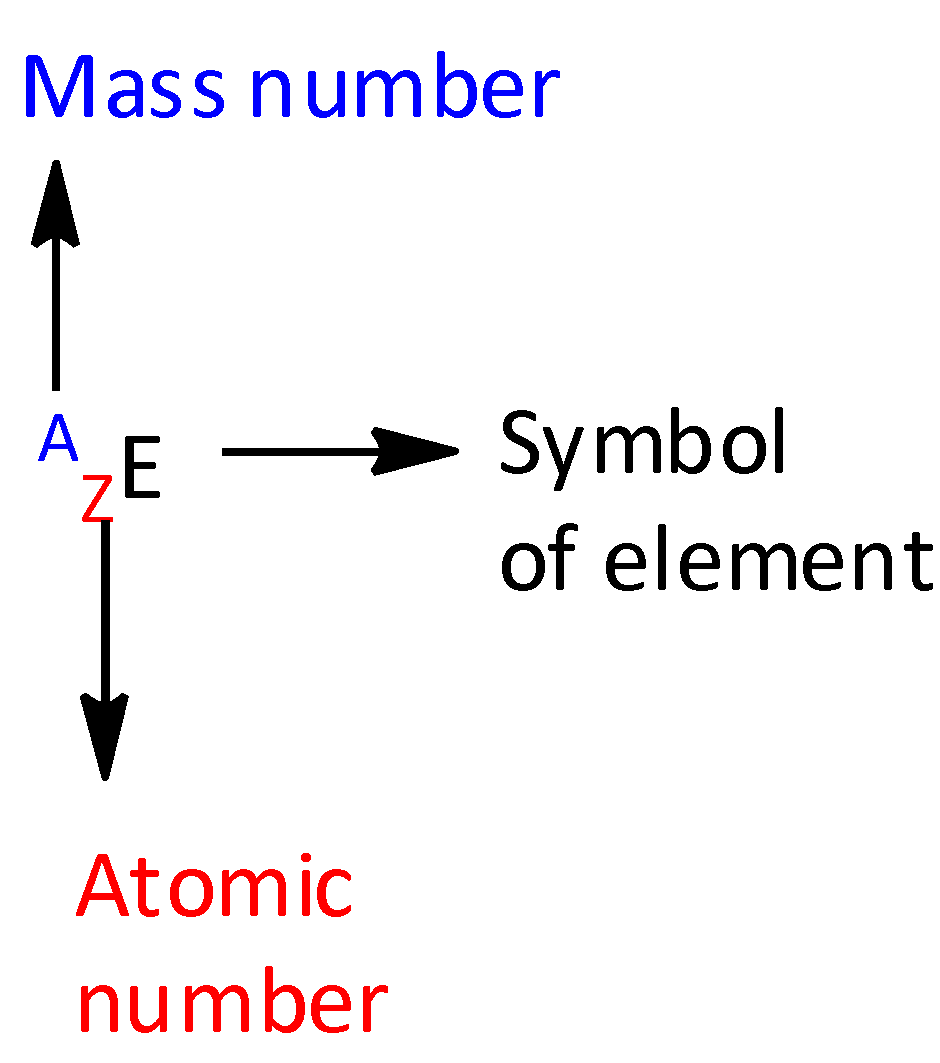
The letter A represents mass number.
The letter Z represents atomic numbers.
Example:
The atomic number of 816O is 8
The mass number of 816O is 16
Atomic number of an element is about the number of protons present.
Atomicnumber=Numberofprotons
The number of protons present will be equal to the number of electrons.
Numberofprotons=Numberofelectrons
Atomic number of 816O is 8. Therefore, the number of protons is 8. The number of electrons is 8.
We can get the number of neutrons by subtracting the mass number and the atomic number
Numberofneutrons=Massnumber−Atomicnumber
The number of neutrons is calculated as,
Number of neutrons = 16−8
Number of neutrons = 8
The number of protons in 816O is 8.
The number of electrons in 816O is 8.
The number of neutrons in 816O is 8.
