Question
Question: Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammonical \({\text...
Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammonical Ni(II). Which of the following statements is not true?
A) Red complex has a tetrahedral geometry
B) Complex has symmetrical H-bonding
C) Red complex has a square planar geometry
D) Dimethylglyoxime functions as bidentate ligand
Solution
Recall the structure of dimethylglyoxime (DMG). It contains two nitrogen atoms and both can donate its electron density to the metal ion. Also, DMG is a strong field ligand therefore, pairing of electrons will occur in the nickel-DMG complex. After pairing, find the hybridisation of nickel-DMG complex and then its geometry.
Complete step by step solution:
We are given that a red precipitate is obtained when ethanol solution of dimethylglyoxime (DMG) is added to ammonical Ni(II). This chemical reaction can be represented as shown below:
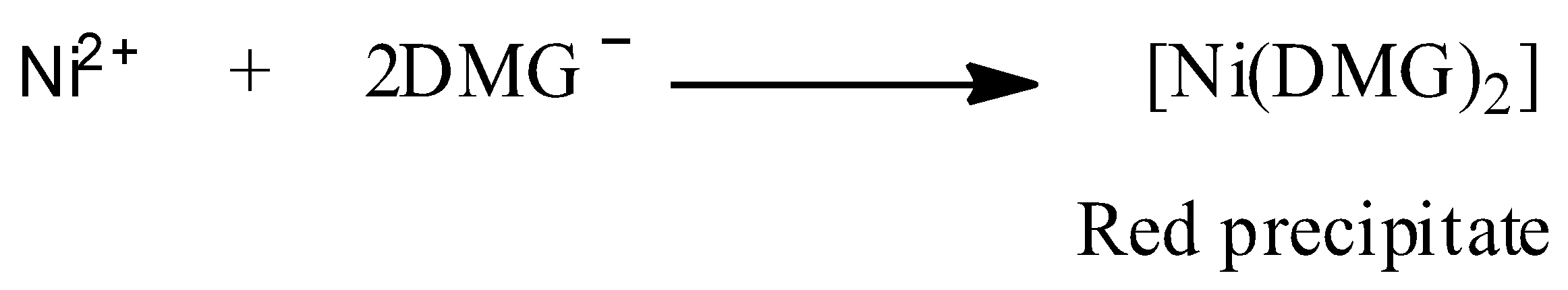
Now, structure of DMG is:
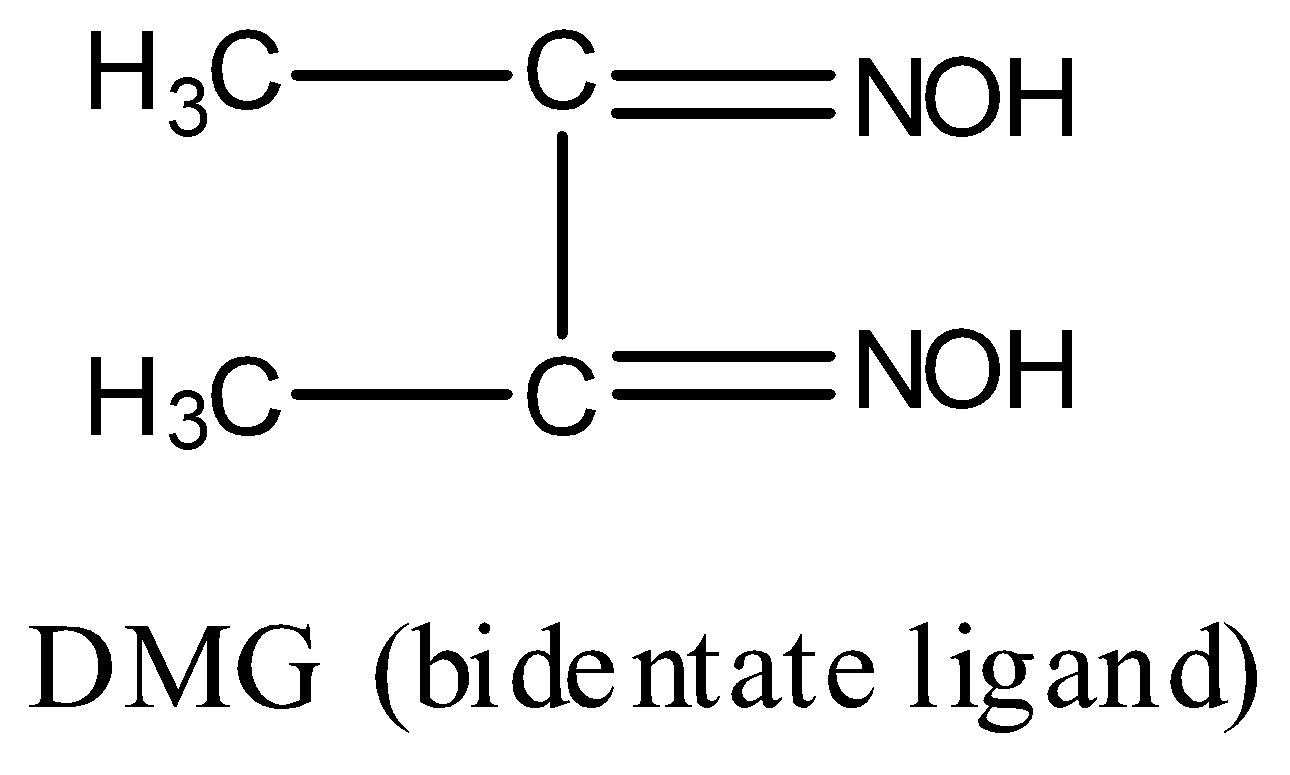
DMG contains two nitrogen atoms and both N will donate electron pairs to the central metal ion, hence, DMG is a bidentate ligand. Thus, the statement given in option D is true.
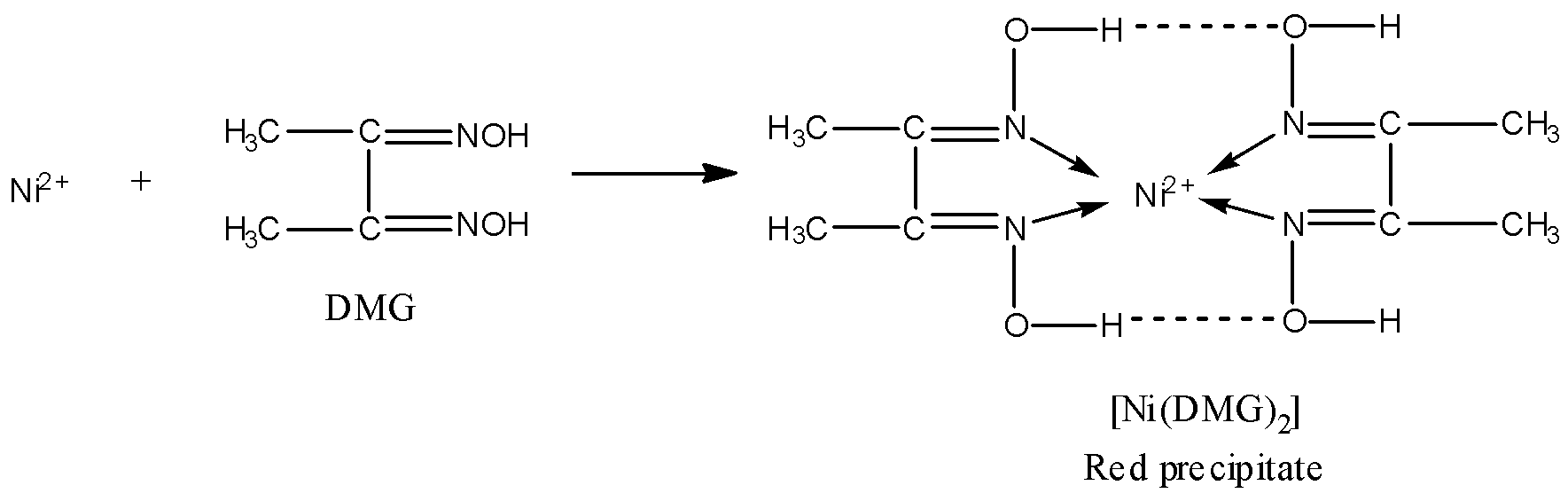
-Now, as you can see in the above nickel-DMG complex that there is symmetrical hydrogen bonding present in the complex. Thus, statement B is also true.
-As you know, Ni is d8 system, therefore:
-Valence electronic configuration of Ni=3d84s2
-Valence electronic configuration of Ni2+=3d8
-Now, we know that dimethylglyoxime (DMG) is a strong field ligand, therefore when nickel is present in complex i.e., [Ni(DMG)2], pairing of d-electrons of Ni2+ will occur. Thus, we can show this as:
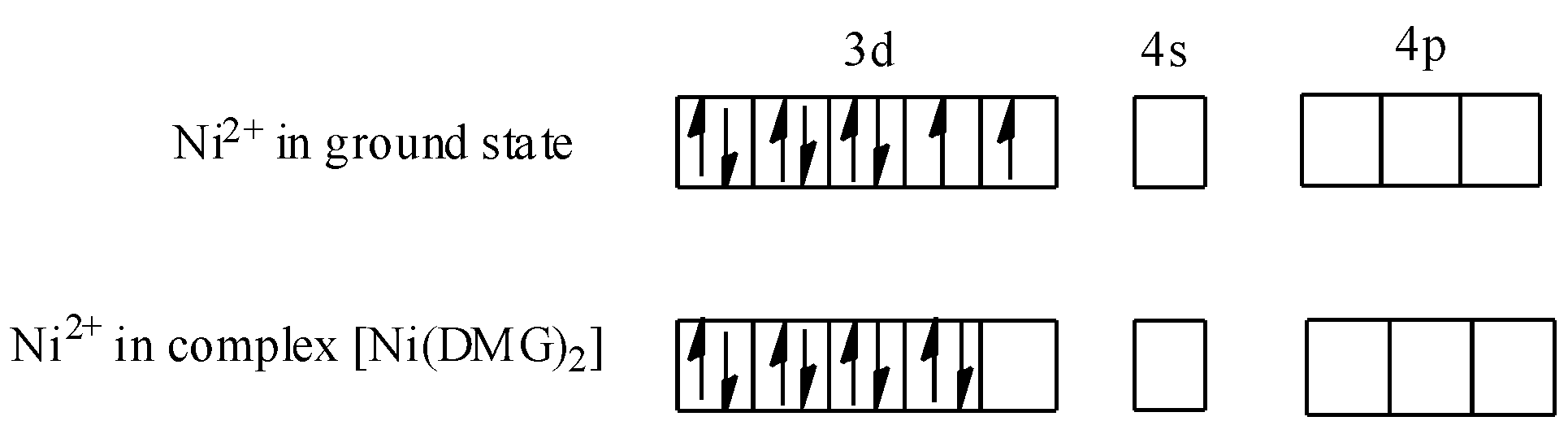
Hence, hybridisation of the [Ni(DMG)2] complex (red complex) will be dsp2 and geometry will be square planar. Thus, statement C is also true. On concluding, we find that the statement given in option A i.e., red complex has a tetrahedral geometry, is not true.
Thus, option A is the answer.
Note: When coordination number of a complex is 4 i.e., four ligands are linked to central metal ions, then the geometry will be either tetrahedral or square planar. For tetrahedral geometry, hybridisation is sp3 whereas for square planar geometry, hybridisation is dsp2.
