Question
Question: Rearrange the following in the correct order of boiling points A. 
B. 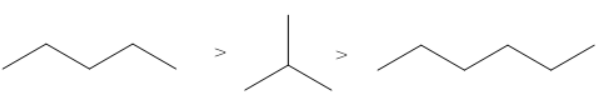
C. 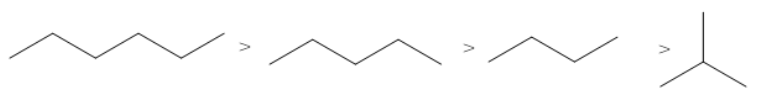
D. 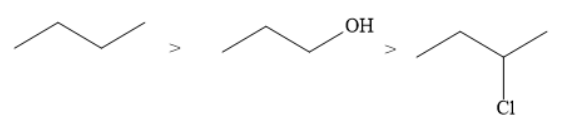
Solution
Consider various factors that affect the boiling points. These include the molecular weight, the degree of branching, presence of hydrogen bonds and dipole-dipole interactions.
Complete answer:
For hydrocarbons, with increase in the number of carbon atoms, the boiling point increases. Also, with increase in branching, the boiling point decreases.
The correct order of the boiling points for the compounds of the option A is
In the above order, isobutane has the lowest boiling point as it contains a branch and the total number of carbon atoms is 4. Butane also contains 4 carbon atoms but has higher boiling point than isobutane as butane lacks branching. Pentane has 5 carbon atoms (maximum number of carbon atoms). Hence, pentane has the highest boiling point.
The correct order of the boiling points for the compounds of the option B is
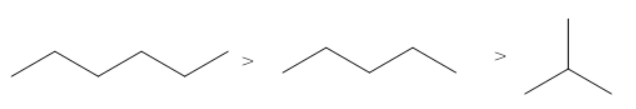
Hexane has the highest boiling point as it has the maximum number of carbon atoms (six carbon atoms). Isobutane has the lowest boiling point as it contains the least number of carbon atoms and a branch.
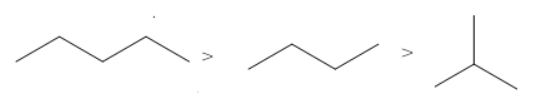
The correct order of the boiling points for the compounds of the option C is
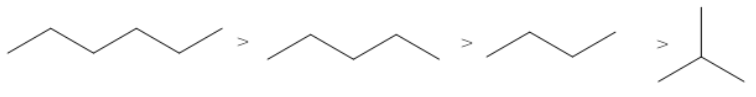
This order is as per the expected order based on the number of carbon atoms and the degree of branching.
The correct order of the boiling points for the compounds of the option D is
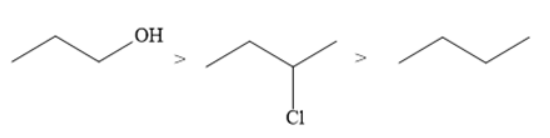
Hydrocarbons have the lowest boiling point when compared to alkyl halides and alcohols. This is because hydrocarbons are non-polar whereas alkyl halides and alcohols are polar. Hydrocarbons contain weak van der Waals forces of attraction. On the other hand, alkyl halides and alcohols contain dipole-dipole forces of attraction that are stronger than van der Waals forces.
Among alkyl halides and alcohols, the maximum boiling point is of alcohols.
Note:
Alcohols have high boiling points due to presence of intermolecular hydrogen bonds. Intermolecular hydrogen bonds lead to molecular association. More energy is needed to break these intermolecular hydrogen bonds. Alkyl halides and alcohols do not form hydrogen bonds.
