Question
Question: Reagent which can convert an alkyl amine into an alkyl chloride. A.Tilden reagent \((NOCl)\) B.L...
Reagent which can convert an alkyl amine into an alkyl chloride.
A.Tilden reagent (NOCl)
B.Lucas reagent
C.Hinsberg’s reagent
D.None
Solution
We need to know that the amine (NH2) is a functional group and also a compound containing a lone pair of nitrogen and it is derived from ammonia. When alkyl groups attached to an amine, that are called alkyl amines, one or more hydrogen atoms are replaced by chloride atoms in an alkyl group called alkyl chlorides.
Complete step by step answer:
We must remember that in order to bring out a chemical reaction, a compound or substance is added, that are called reagents. To measure, detect, examine or to produce other compounds in a chemical reaction is used by a substance or compound is called chemical reagents.
Let us see the options one by one to convert an alkyl amine into an alkyl chloride,
A.Nitrosyl chloride (also known as Tilden’s reagent)
Tilden’s reagent is a yellow gas with a molecular formula (NOCl) and it is prepared by dehydration of nitrous acid by hydrogen chloride.
Alkyl amine reaction with nitrosyl chloride is,
R−NH2+NOCl→R−Cl+NO−NH2
R is an alkyl group in the above reaction.
B.Differentiation of an alcohols by Lucas test
Lucas reagent is a mixture of concentration of hydrochloric acid and zinc chloride. It is used to differentiate alcohol.
R−OH+HClanhydrousZnCl2RCl+H2O
C.Hinsberg’s reagent
Benzene sulphonyl chloride is also called as Hinsberg’s reagent. The chemical reaction
Of Hinsberg’s test is,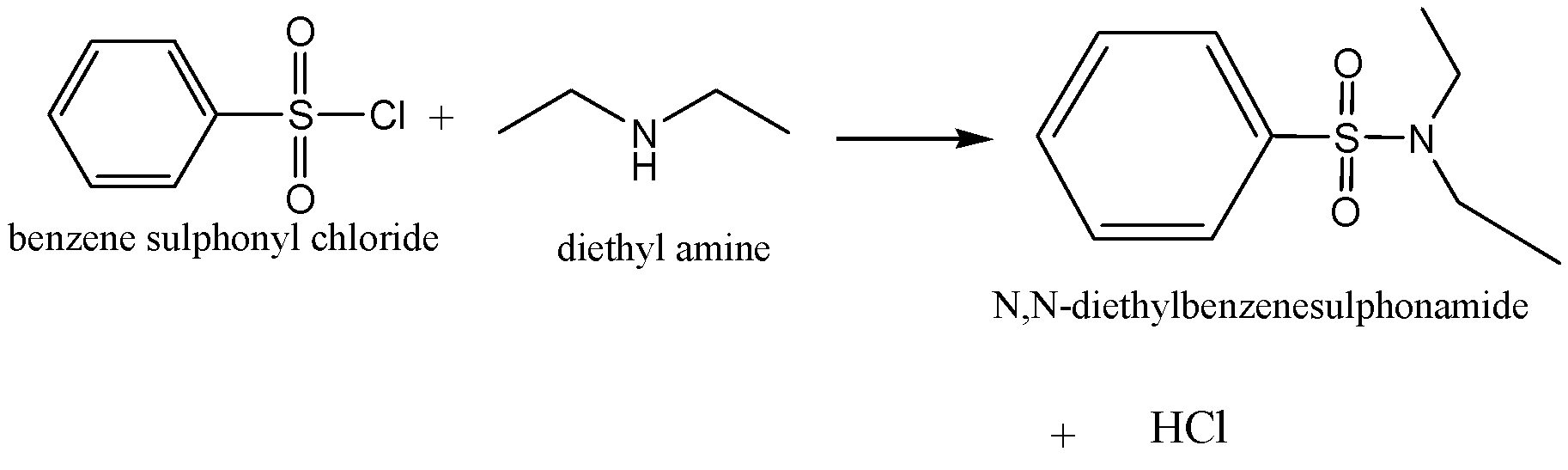
From the above information, an alkyl amine is converted to an alkyl chloride by Tilden reagent (NOCl) . The chemical reaction is,
R−NH2+NOCl→RCl+NO−NH2
So, the correct answer is “Option A”.
Note:
We must remember that Tilden's reagent should be used safely when using it, because nitrosyl chloride is a very toxic reagent. And it is irritating to the eyes, skin and lungs. In industry, nitrosyl chloride is used to form cyclohexanone oxime by reaction with cyclohexane, photo dissociation of this process gives NO&Cl radicals, and the oxide is a precursor of nylon−6 .
