Question
Question: Reagent "P" in the given reaction is : ...
Reagent "P" in the given reaction is :
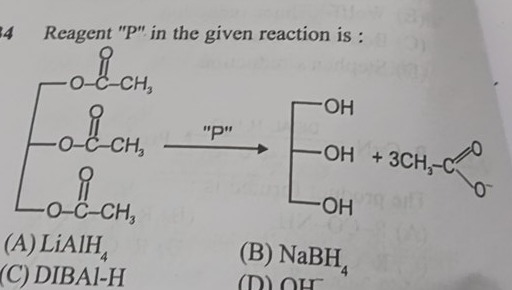
LiAlH4
NaBH4
DIBAI-H
OH−
OH−
Solution
The given reaction involves the conversion of glycerol triacetate (a triester) into glycerol (a triol) and acetate ions. This transformation is a hydrolysis reaction, specifically the base-catalyzed hydrolysis of an ester, also known as saponification. In this process, the ester bond is cleaved by a nucleophilic attack of a hydroxide ion (OH−) on the carbonyl carbon, leading to the formation of an alcohol and a carboxylate salt.
-
LiAlH4 (Lithium Aluminum Hydride): This is a strong reducing agent that reduces esters to primary alcohols, not acetate ions.
-
NaBH4 (Sodium Borohydride): This is a milder reducing agent that typically reduces aldehydes and ketones but generally does not reduce esters.
-
DIBAI-H (Diisobutylaluminum hydride): This is a reducing agent that can reduce esters to aldehydes or primary alcohols, not acetate ions.
-
OH− (Hydroxide ion): A hydroxide ion, typically supplied by a strong base like NaOH or KOH, is the characteristic reagent for the base-catalyzed hydrolysis (saponification) of esters.
Therefore, reagent "P" is OH−.
