Question
Question: Reaction of t-butyl bromide with sodium methoxide produces: (A) Isobutane (B) Isobutylene (C) ...
Reaction of t-butyl bromide with sodium methoxide produces:
(A) Isobutane
(B) Isobutylene
(C) Sodium- t- butoxide
(D) t- butyl methyl ether.
Solution
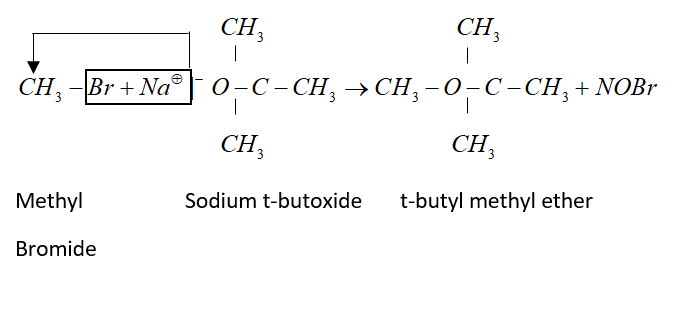
This method is called Williamson’s synthesis. General reaction of this synthesis is:
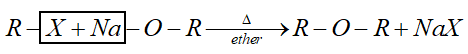
Complete step by step answer:
When alkyl halide is heated with alc. Sodium or potassium alkoxide gives corresponding ethers. Simple ethers can be easily prepared by this method.
But for preparation of mixed ether a proper choice of reactant is necessary. Primary alkyl halide is more susceptible to SN2 reaction. Therefore, best yield of ether obtained when alkyl halide is primary and alkoxide is tertiary.
Example: tert-butyl ether is prepared by heating ethyl bromide with sodium tert-butoxide.
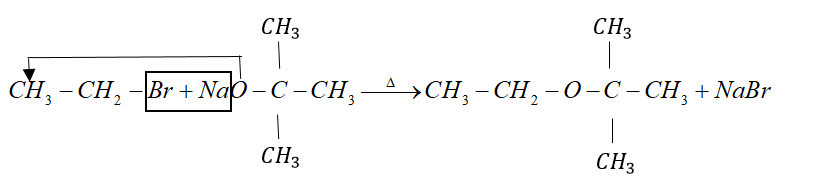
But when alkyl halide is secondary or tertiary the nucleophilic attack of alkoxide ion on α− carbon atom becomes difficult due to crowding effect.
Since alkoxide is a stronger base and attacking β− hydrogen is easier. Therefore β− elimination dominates.
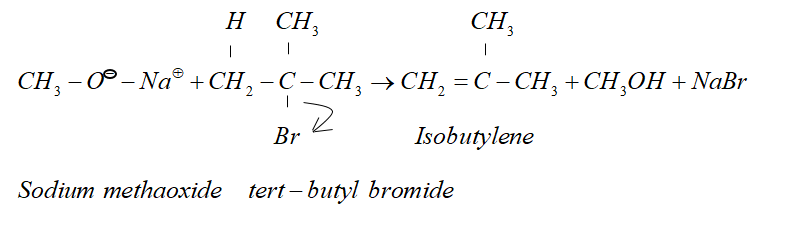
Therefore, in the given reaction isobutylene is formed.
So, the correct answer is “Option B”.
Note:
Williamson’s synthesis is a good method for preparation of mixed ether. But proper choice of reagent should be necessary. If we want to prepare t-butyl methyl ether then methyl bromide and sodium t-butoxide should be taken.