Question
Question: Reaction of formaldehyde and ammonia gives: A. hexamethylenetetramine B. Bakelite C. urea D....
Reaction of formaldehyde and ammonia gives:
A. hexamethylenetetramine
B. Bakelite
C. urea
D. triethylenetetramine
Solution
Action of Formaldehyde on Ammonia is an example of hetero condensation polymer. Polymers are large molecules made by the linkage of small units. Condensation polymers are accompanied by loss of substances like water from the reaction.
Complete step by step answer:
Formaldehyde HCHO reacts with ammonia NH3 in neutral or alkaline solution. Due to the basic medium, neutralisation also takes place when formaldehyde reacts with ammonia by heating up ammonia bicarbonate or ammonium carbonate to generate ammonia vapours. The ammonia vapours formed neutralise the formaldehyde gas which creates a by-product called methenamine. Methenamine is also called hexamine, urotropin and hexamethylenetetramine. The structure of methenamine along with reaction is
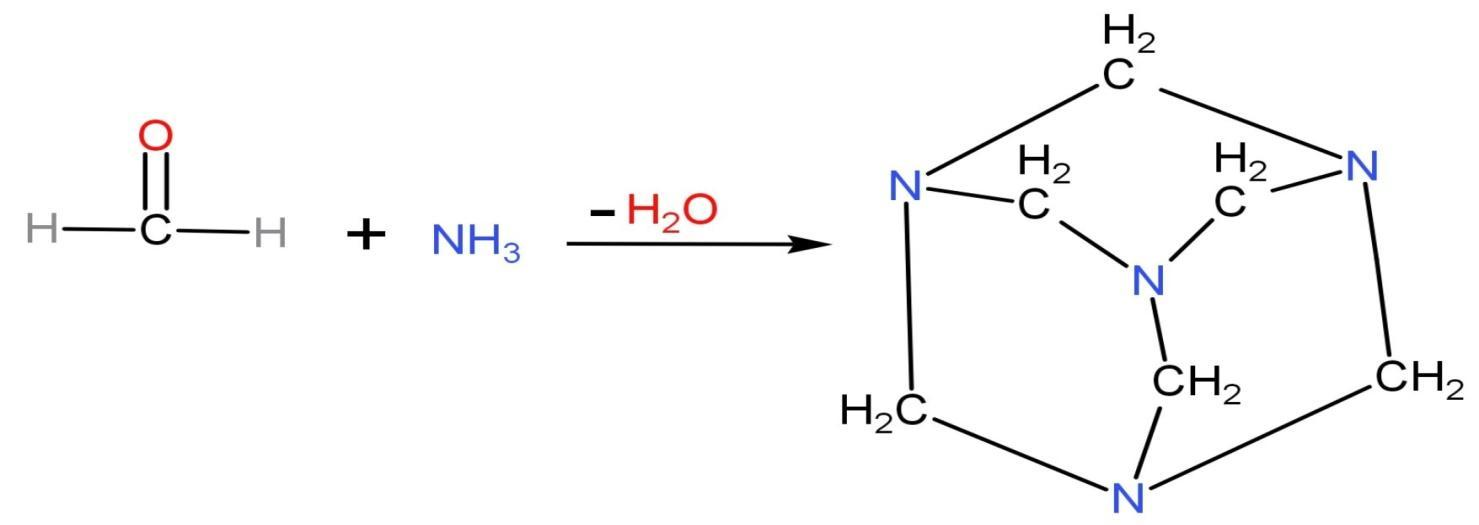
Hexamethylenetetramine is a heterocyclic organic compound which has molecular formula (CH2)6N4. It is a white crystalline compound which is highly soluble in water and polar organic solvents. It has a cage-like structure.
The correct answer is option A.
Additional Information:
Uses of hexamethylenetetramine:
(1) Hexamine is also used as a food additive as a preservative.
(2) Methenamine in the form of cream and spray is used for treatment of excessive sweating and odour.
(3) The use of hexamethylenetetramine is in the production of liquid preparations of phenolic resins and its moulding compounds, where it is used as a hardening component.
Note:
The point to note in this reaction is that this reaction is reversible. It is an interconvertible reaction. By adding OH− ions, the reaction will proceed forward and addition of H+ ions, the reaction will move backward. 6HCHO+4NH3⟶C6H12N4+6H2O
