Question
Question: Reaction of ethyl formate with an excess of \[C{H_3}MgI\] followed by hydrolysis given: A.N- propy...
Reaction of ethyl formate with an excess of CH3MgI followed by hydrolysis given:
A.N- propyl alcohol
B.Isopropyl alcohol
C.Acetaldehyde
D.Acetone
Solution
Ethyl format is an ethyl ester of formic acid. Its microwave spectrum indicates the presence of two isomeric forms having the ethyl group cis to the carbonyl oxygen atom but having different arrangement of the methyl group about the −CH2−O bond. Kinetics of its pyrolytic decomposition to the corresponding acid and alkene has been reported.
Complete step by step:
Here ethyl acetate reacts with an excess of CH3MgI and followed by hydrolysis which is given below reaction,
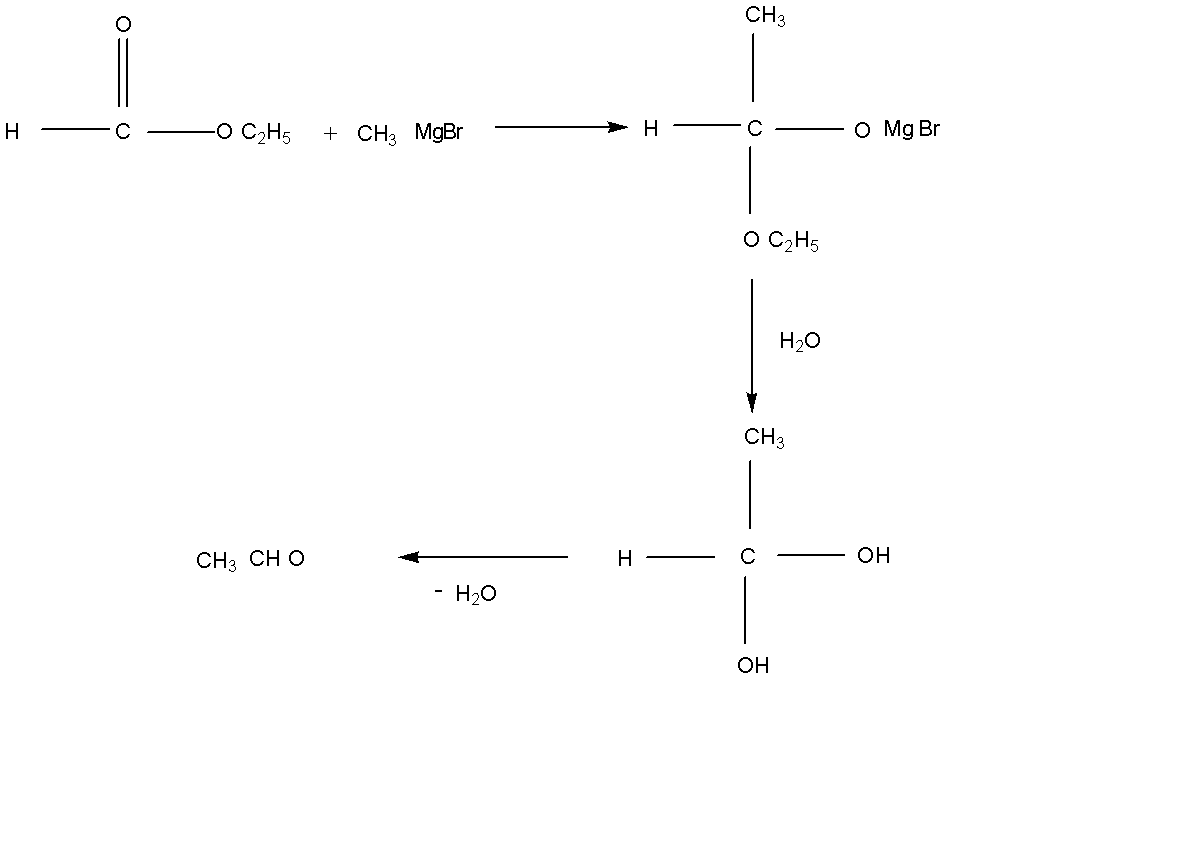
Here we see the reaction between ethyl formate with an excess CH3MgI and given us n propyl alcohol as a product.
Hence option (A) is the correct answer.
Additional information: Ethyl formate is used industrially as a solvent and as a fungicide and parricide in processed foods such as dried fruits and cereals. In pure form, Ethyl formate has several dangerous properties as shown in the hazard information box. It is a simple aliphatic ester with dull benefits and concerns. It’s a clear, slightly oily liquid with a pleasant, rum like odor and the flavor of raspberries.
Note: Ethyl formate reacts with CH3MgI in excess condition by the hydrolysis reaction. Water molecules are part of the reaction and formed the medium product. After that water molecule was removed as a water vapor and formed the product which is n propyl alcohol. Here we remember that in the first step a water molecule is added in the reaction for the hydrolysis reaction.
