Question
Question: Reaction: \[ 3A \rightarrow 2B + C \] Given: 1. The reaction follows a complex rate law: Rate = k...
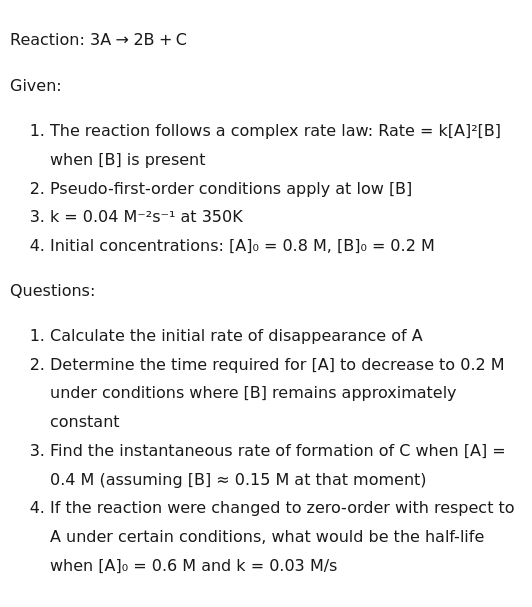
Reaction: 3A→2B+C
Given:
- The reaction follows a complex rate law: Rate = k[A]²[B] when [B] is present
- Pseudo-first-order conditions apply at low [B]
- k = 0.04 M⁻²s⁻¹ at 350K
- Initial concentrations: [A]₀ = 0.8 M, [B]₀ = 0.2 M
Questions:
- Calculate the initial rate of disappearance of A
- Determine the time required for [A] to decrease to 0.2 M under conditions where [B] remains approximately constant
- Find the instantaneous rate of formation of C when [A] = 0.4 M (assuming [B] ≈ 0.15 M at that moment)
- If the reaction were changed to zero-order with respect to A under certain conditions, what would be the half-life when [A]₀ = 0.6 M and k = 0.03 M/s
- Initial rate of disappearance of A: 0.01536Ms−1
- Time required for [A] to decrease to 0.2 M (constant [B]): 156.25s
- Instantaneous rate of formation of C when [A] = 0.4 M ([B] = 0.15 M): 0.00096Ms−1
- Half-life for zero-order A: 10s
Solution
The problem presents a chemical reaction and a complex rate law, along with specific conditions for different parts of the question. A significant inconsistency arises from B being a product in the stoichiometric equation (3A→2B+C) but appearing as a reactant in the rate law (Rate = k[A]2[B]). Furthermore, its concentration behavior is specified in contradictory ways across different parts. We will proceed by strictly adhering to the conditions and values provided for each sub-question, acknowledging these inconsistencies.
Given Information:
- Reaction: 3A→2B+C
- Rate Law: Rate = k[A]2[B] (This is the rate of reaction, Rate=−31dtd[A]=+21dtd[B]=+dtd[C])
- Rate Constant: k=0.04M−2s−1 at 350K
- Initial Concentrations: [A]0=0.8M, [B]0=0.2M
1. Calculate the initial rate of disappearance of A
The initial rate of reaction is given by the rate law using initial concentrations: Initial Rate=k[A]02[B]0 Substitute the given values: Initial Rate=(0.04M−2s−1)(0.8M)2(0.2M) Initial Rate=(0.04)(0.64)(0.2)Ms−1 Initial Rate=0.00512Ms−1 The rate of disappearance of A is related to the rate of reaction by the stoichiometry: −dtd[A]=3×Rate Initial Rate of disappearance of A=3×0.00512Ms−1 Initial Rate of disappearance of A=0.01536Ms−1
2. Determine the time required for [A] to decrease to 0.2 M under conditions where [B] remains approximately constant
If [B] remains approximately constant, the rate law simplifies to a pseudo-second-order reaction with respect to A. We use the initial concentration of B for this calculation. The rate of disappearance of A is: −dtd[A]=3×Rate=3k[A]2[B] Let k′=3k[B]. Since [B] is constant (at [B]0=0.2M), k′ is a constant: k′=3×(0.04M−2s−1)×(0.2M) k′=0.024M−1s−1 The integrated rate law for a second-order reaction (with respect to A) is: [A]t1−[A]01=k′t We want to find t when [A]t=0.2M, with [A]0=0.8M: 0.2M1−0.8M1=(0.024M−1s−1)t 5M−1−1.25M−1=(0.024M−1s−1)t 3.75M−1=(0.024M−1s−1)t t=0.0243.75s t=156.25s
3. Find the instantaneous rate of formation of C when [A] = 0.4 M (assuming [B] ≈ 0.15 M at that moment)
The instantaneous rate of formation of C is equal to the rate of reaction (+dtd[C]=Rate). We use the given instantaneous concentrations for [A] and [B]. Rate=k[A]2[B] Substitute the given values: Rate=(0.04M−2s−1)(0.4M)2(0.15M) Rate=(0.04)(0.16)(0.15)Ms−1 Rate=0.04×0.024Ms−1 Rate=0.00096Ms−1 Therefore, the instantaneous rate of formation of C is 0.00096Ms−1.
4. If the reaction were changed to zero-order with respect to A under certain conditions, what would be the half-life when [A]₀ = 0.6 M and k = 0.03 M/s?
For a zero-order reaction with respect to A, the rate of disappearance of A is constant: −dtd[A]=kzero The given rate constant k=0.03M/s has units consistent with a zero-order rate of disappearance of A. The integrated rate law for a zero-order reaction is: [A]t=[A]0−kzerot The half-life (t1/2) is the time when [A]t=[A]0/2: 2[A]0=[A]0−kzerot1/2 kzerot1/2=[A]0−2[A]0 kzerot1/2=2[A]0 t1/2=2kzero[A]0 Substitute the given values: t1/2=2×(0.03M/s)0.6M t1/2=0.060.6s t1/2=10s
Explanation of the solution:
- Initial Rate of Disappearance of A: Calculated the initial reaction rate using the given rate law and initial concentrations. Then, multiplied by the stoichiometric coefficient of A (3) to find its disappearance rate.
- Time for [A] to Decrease (Constant [B]): Assumed [B] remains constant at its initial value as specified. The rate law becomes pseudo-second-order with respect to A. Applied the integrated rate law for a second-order reaction to find the time.
- Instantaneous Rate of Formation of C: Used the given instantaneous concentrations of [A] and [B] directly in the rate law. The rate of reaction equals the rate of formation of C.
- Half-life for Zero-Order A: Applied the half-life formula for a zero-order reaction, t1/2=2k[A]0, using the provided initial concentration and zero-order rate constant.
