Question
Question: \(RCH = C{H_2}\xrightarrow[{\left( 1 \right){O_2}}]{{\left( 2 \right){H_2}O/Zn}}\left( A \right)\xri...
RCH=CH2(2)H2O/Zn(1)O2(A)LiAlH4(B)
What is B?
A. RCHO+HCHO
B. RCHO+HCOOH
C. RCOOH+HCOOH
D. RCH2OH+CH3OH
Solution
When alkene reacts with oxygen and zinc, ozonolysis takes place. In this reaction the double bond of the alkene breaks and alkene is converted into aldehyde. Hydrocarbons that contain −OH functional groups are called aldehydes.
Complete answer:
In this question we have given a series of reactions and we have to find the final product. First step of the reaction is:
RCH=CH2(2)H2O/Zn(1)O2(A)
In this reaction, the given alkene (RCH=CH2) will react with oxygen and then with water and zinc. Reactions in which alkene first react with oxygen and then with water in the presence of zinc , ozonolysis of alkene takes place. These two steps combine and ozonolysis of reactant takes place. Hence reactants react with ozone in the presence of reducing agents such as zinc. Now, the reaction is:
RCH=CH2+O3Zn(A)
In this reaction double bond between the carbon atom breaks and formation of aldehyde takes place. Mechanism of this reaction is as follows:
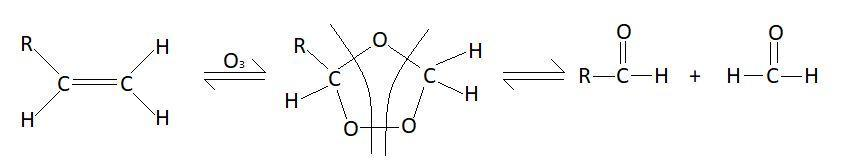
So, the products of this reaction will be:
RCH=CH2+O3ZnRCHO+CH2O
These are the products of the first step of the reaction. Now the second step of reaction is:
RCHO+CH2OLiAlH4(B) (Obtained by substituting products of first reaction)
Given reactants are aldehydes. Lithium aluminium hydride (LiAlH4) is a reducing agent. It can reduce aldehyde to alcohol. This means given aldehydes will be converted to alcohols. Corresponding alcohol of RCHO will be RCH2OH and that of CH2O will be CH3OH. So, the products of given reaction are:
RCHO+CH2OLiAlH4RCH2OH+CH3OH
So, the correct answer is option D.
Note:
In ozonolysis reaction if the reactant is alkene (hydrocarbon with double bond between carbon atoms) then the product is aldehyde and if the reactant is alkyne (hydrocarbon with triple bond between carbon atoms) then the product is ketone.
