Question
Question: Raw material for the production of urea are: A. ammonia and carbon dioxide B. oxygen and car...
Raw material for the production of urea are:
A. ammonia and carbon dioxide
B. oxygen and carbon dioxide
C. ammonia and oxygen
D. ammonia and phosphate
Solution
Urea has an Amide functional group. Urea is diamide of carbonic acid. When both hydroxyl groups of carbonic acid are replaced with ammonia groups, the formed structure is known as amide. The structure or constitutes of the product gives the idea about reactants.
Complete step by step solution: The structure of urea is as follows:
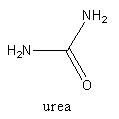
The structure tells that urea contain amine and carbonyl functional groups. The reactant for urea preparation will be ammonia and carbon dioxide. At industrial level, urea is prepared by reacting ammonia with carbon dioxide.
Urea formed at high temperature and pressure. Formation take place in two steps:
First, ammonia and carbon dioxide reacts to give ammonium carbonate. It is a reversible step.
The reaction of formation of ammonia carbonate is as follows:
NH3 + CO2⇄NH2COONH4
NH2COONH4 is known as ammonium carbonate.
In the second step, ammonium carbonate decomposes to give urea and water. It is also a reversible step.
NH2COONH4⇄NH2COONH2 + H2O
By the same reaction ammonia and carbon dioxide can be recycled. On decreasing pressure and increasing the temperature, ammonium carbonate decomposes into ammonia and carbon dioxide.
So, raw materials for the production of urea are ammonia and carbon dioxide.
Therefore, option (A) ammonia and carbon dioxide, is correct.
Note: Urea has a high amount of nitrogen, so it is used as fertilizer. In the laboratory, urea is prepared by the Wohler method. Urea is also used in manufacturing of resin. Ammonia is the main reactant for the urea production, so urea manufacturing is done nearby ammonia production plants. At industry level, ammonia is produced by the Haber process. In the Haber process, nitrogen is reacted with hydrogen at high temperature in presence of a catalyst to produce ammonia.
