Question
Question: Ratio of hydrogen and oxygen of water molecules. A. 1:2 B. 2:3 C. 2:1 D. 3:2...
Ratio of hydrogen and oxygen of water molecules.
A. 1:2
B. 2:3
C. 2:1
D. 3:2
Solution
Hint: It is already known to us that the chemical formula of water is and the ratio of hydrogen to oxygen atoms will always remain the same, no matter how many number of water molecules are taken.
Complete step by step solution:
Whenever H2O is produced by combining hydrogen and oxygen gas, then it always takes two molecules of diatomic hydrogen gas and one molecule of oxygen gas to produce two molecules of water. The chemical reaction which takes place is:
2H2+O2→2H2O
In other words, from the above chemical reaction, we can say that the ratio of hydrogen to oxygen is 2:1, the ratio of hydrogen to water is 1:1 and the ratio of oxygen to water is 1:2.
Therefore, from above it can be easily said that option C is the correct answer.
Additional Information:
The hybridization of H2O molecule is sp3 and from hybridization we can easily say that the geometry of H2O molecule is tetrahedral and the structure of H2O is bent shape in gaseous phase. The geometry of water is
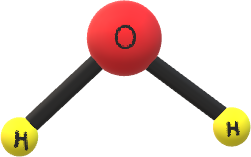
Also, water has a higher specific heat, thermal conductivity and surface tension compared to other liquids.
Also, natural water contains many dissolved salts and depending upon their behavior towards soap solution i.e. lather formation, they are classified as soft water and hot water.
Note: It should be kept in mind that most of the unique properties of water are due to the presence of hydrogen bonding between its molecules.
Also, it should be remembered that water is amphoteric in character i.e. can act both as an acid and as a base.
