Question
Question: Rank the following substances in order of decreasing heat of combustion (maximum – minimum): :
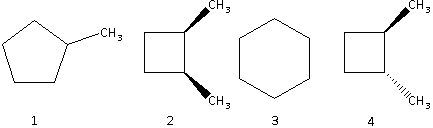
A. 1>2>3>4
B. 3>4>2>1
C. 2>4>1>3
D. 1>3>2>4
Solution
The heat of combustion is inversely proportional to the stability of the ring. Thus,
Heat of combustion∝Stability of the ring1.
Complete step by step answer:
Step 1:
Determine the order of stability as follows:
The stability of the ring increases as the size of the ring increases. Substance 2 and substance 4 have the same ring size. The size of substance 1 is greater than substance 2 and 4 and the size of substance 3 is the greatest.
Thus, the order of stability is 3>1>2=4.
The substances 2 and 4 are isomers. The trans isomer is more stable than the cis isomer. This is because in a trans isomer, the substituent groups are attached on the opposite sides of the carbon – carbon bond which decreases the strain in the ring resulting in an increased stability. Thus, the substance 4 (trans isomer) is more stable than substance 2 (cis isomer).
Thus, the order of stability is 3>1>4>2.
Step 2:
Determine the order of heat of combustion as follows:
The heat of combustion is inversely proportional to the stability of the ring. Thus,
Heat of combustion∝Stability of the ring1
Thus, the order of heat of combustion is the opposite of the order of the stability of the ring.
Thus, the order of heat of combustion is 2>4>1>3.
Thus, the correct option is option (C).
Note: In a trans isomer, the substituent groups are attached on the opposite side of the carbon – carbon bond. In a cis isomer, the substituent groups are attached on the same side of the carbon – carbon bond. As a result, the steric hindrance due to the substituent groups decreases in a trans isomer. Thus, the trans isomer is always more stable than the cis isomer.
