Question
Question: Rank the bond dissociation energies of the bonds indicated with the arrows. (From smallest to larges...
Rank the bond dissociation energies of the bonds indicated with the arrows. (From smallest to largest).
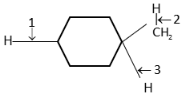
A. 1<2<3
B. 3<2<1
C. 2<3<1
D. 3<1<2
Solution
Bond dissociation energy is a measure of strength of bonds between two atoms and the energy is required to break one mole of a bond of a particular type between the atoms in gaseous state under standard conditions.
More the bond dissociation energy, the stronger the bond is. This is used in case of heterolytic cleavage. If the bond cleavage leads to the formation of stable products, it will require less bond dissociation energy. This is used in homolytic cleavage. We will use this concept to solve this question.
Complete step-by-step answer: In this molecule,
the dissociation of the C-H bond would result in the formation of a radical. By comparing the radical stability, we will be able to compare the bond dissociation energy. We know that the primary free radical is least stable and the tertiary free radical is the most stable because the tertiary free radical is most stable due to the +I effect (inductive effect) of two alkyl groups and due to hyperconjugation.
Since tertiary radical is most stable and hence the bond between tertiary carbon and hydrogen can be broken easily into tertiary radical and hence will require least bond dissociation energy.
Hence, dissociation of a primary carbon hydrogen bond will be most difficult, i.e., require more energy. In case of tertiary carbon, the energy required will be least.
Therefore, order of bond dissociation energy is3<1<2 because stability of free radicals follows the following order.
tertiary carbon free radical>secondary free radical>primary free radical$.
Hence, the correct option is (D).
Note: Bond can be broken symmetrically or asymmetrically. The former is called homolytic cleavage and is the basis of the usual bond dissociation energies. Asymmetric dissociation of a bond is called heterolytic cleavage but in gaseous phase, the enthalpy of heterolytic cleavage is larger than homolytic cleavage, due to the need to separate unlike charges. Both homolytic and heterolytic cleavage are thermodynamically endothermic reactions.
