Question
Question: \( R - N{H_2} + CHC{l_3} + 3KOH \to A + 3KCl + 3{H_2}O \) . A is (A) \( R - NC \) (B) \( R - N{...
R−NH2+CHCl3+3KOH→A+3KCl+3H2O . A is
(A) R−NC
(B) R−NH2
(C) R−NO2
(D) All
Solution
Hint : When primary amine, chloroform and potassium hydroxide react it will lead to the formation of isocyanide. This reaction is known as carbylamine reaction. This reaction completed with the formation of dichlorocarbene as an important intermediate product.
Complete Step By Step Answer:
Aliphatic and aromatic primary amines when warmed in the presence of chloroform and an alcoholic solution of potassium hydroxide, form isocyanide. Isocyanide is also known as carbylamine which has a characteristic foul smell.
Secondary and tertiary amines are not undergoing this test therefore this reaction is widely used to distinguish primary aliphatic or aromatic amines from secondary and tertiary amines.
The reaction proceeds with the addition step of primary amine to reaction intermediate dichlorocarbene (CCl2) .
Dichlorocarbene is generated when chloroform undergoes dehydrohalogenation to remove hydrogen and one halogen atom.
In the first step dichlorocarbene attack the primary amine to generate unstable intermediate
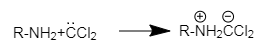
This intermediate further reacts with hydroxide ion generated by potassium hydroxide followed by a rearrangement process to produce isocyanide.
During the complete reaction intermediate is attacked twice by the hydroxide ion to complete the chemical reaction.
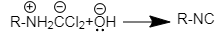
Carbylamine reaction will also lead to the formation of three molecules of potassium chloride and three molecules of water as a byproduct.
Hence, the complete balanced chemical reaction become
R−NH2+CHCl3+3KOH→R−NC+3KCl+3H2O
Therefore the option (A) is the correct option.
Note :
Carbylamine reaction is commonly known as Hofmann isocyanide synthesis reaction. Dichlorocarbene is also formed as an intermediate during the Reimer-Tiemann reaction.
Due to the presence of a triple bond between the nitrogen and carbon atom in isocyanide (N≡C) it will readily undergo polymerization reactions.
