Question
Question: \({R_3}SiCl\) on hydrolysis forms (A) \({R_3}SiOH\) (B) \({R_3}Si - O - Si{R_3}\) (C) \({R_...
R3SiCl on hydrolysis forms
(A) R3SiOH
(B) R3Si−O−SiR3
(C) R2Si=O
(D) 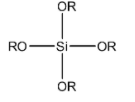
Solution
Hydrolysis is the reaction in which a bond in the substrate is broken by its reaction with water. Generally, hydroxyl groups of water get connected to the electropositive atom of the bond and hydrogen atom of water forms a bond with electronegative atoms of the bond.
Complete step by step solution:
We will know about the hydrolysis reaction.
- Hydrolysis is the reaction by which a bond in the substrate molecule is broken by its reaction with water.
- The given substrate molecule is R3SiCl.
- Here, the most polar bond is the Si-Cl bond. This is because Cl is a highly electronegative element because it is a halogen. Silicon has relatively the same electronegativity as carbon. Thus, we can say that chlorine has partial negative charge and silicon has partial positive charge in the bond.
- We know that water has −OH as a nucleophilic part and H+ as an electrophilic part. So, the reaction can be shown as below with mechanism.
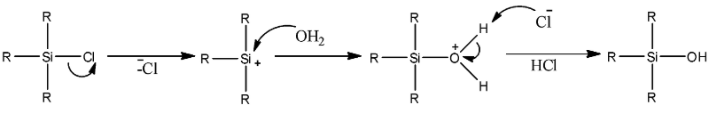
- Here, we can see that first a cation is formed. Then this cation is attacked by nucleophilic oxygen atoms of water. This leads to the formation of a silicon compound having –OH group. Thus, the final product we obtain is R3Si−OH.
- Here, if we further heat the solution, then a condensation reaction would occur and that would lead to the formation of the compound R3Si−O−SiR3 and a water molecule.
- Thus, we can say that R3SiCl on hydrolysis forms R3Si−OH.
So, the correct answer to this question is (A).
Note: Alkyl or aryl substituted silicon chlorides have the common formula RnSiCl4−n and they are collectively known as silicones. They are used as sealants, electrical insulators and in the manufacture of waterproofing fabrics and grease.
