Question
Question: (R)-\(2\)-octyl tosylate is solvolysis in water under ideal \({S_N}1\) conditions. The product(s) wi...
(R)-2-octyl tosylate is solvolysis in water under ideal SN1 conditions. The product(s) will?
a.) R-2-octanol and S-2-octanol(excess)
b.) R-2-octanol and S-2-octanol
c.) R-2-octanol only
d.) S-2-octanol only
Solution
To solve this problem we should know about the R and S form of the structures given in question. Here in solvation water will act as a nucleophile during the reaction. We will consider ideal SN1 conditions.
Complete step by step answer:
First we will draw the structure of reactant (R)-2-octyl tosylate and will understand about the R and S form.
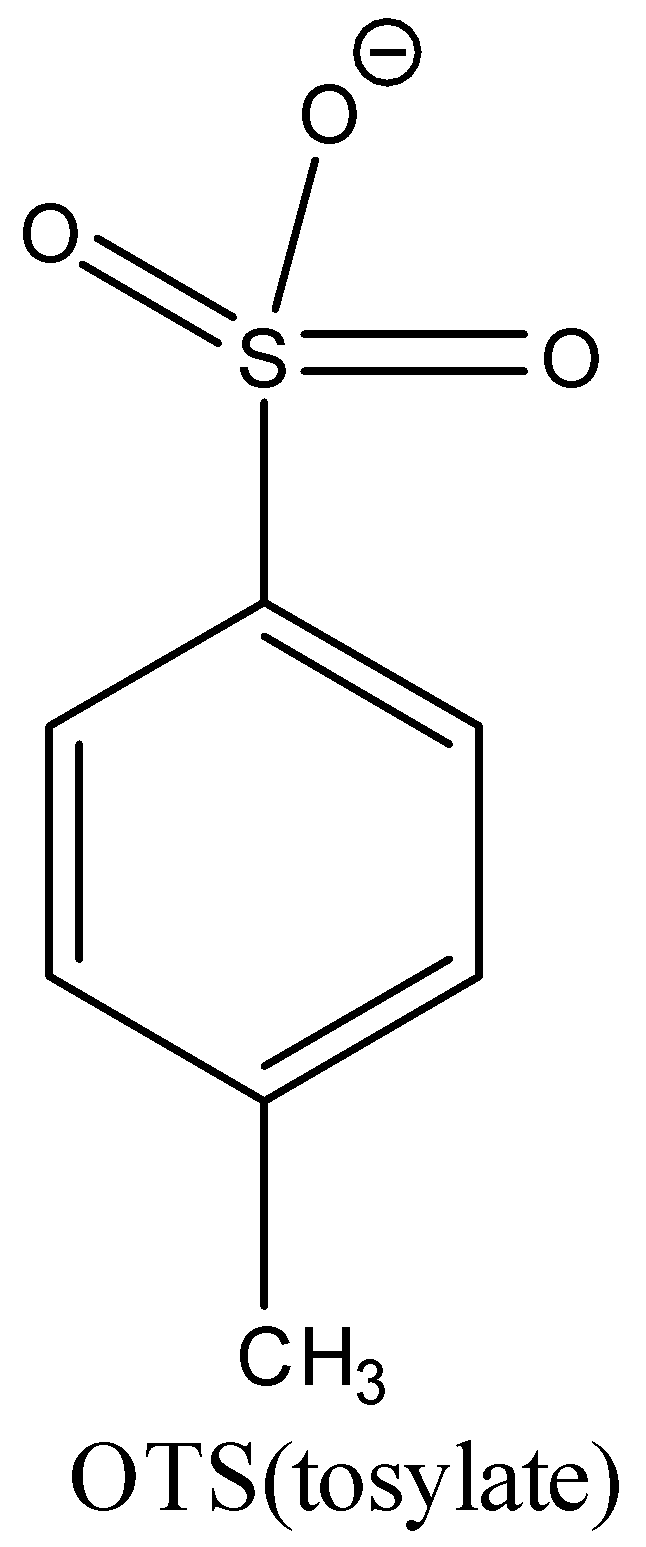
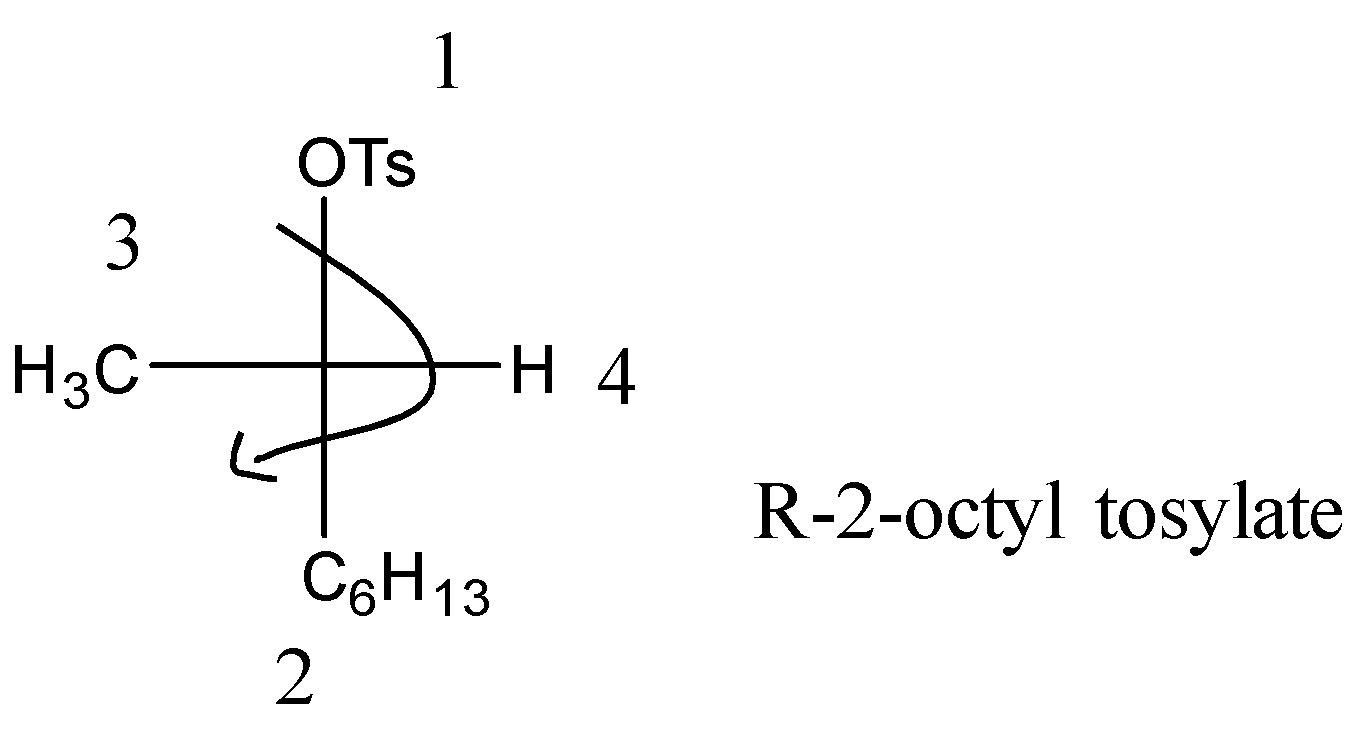
The above structure is tosylate(OTs) but the given reactant is (R)-2-octyl tosylate so the reactant’s structure is on right with the octyl group at C-2 position.
- Now we will understand the concept of R and S form. First we will number the chemical compounds in the order of higher molecular mass. For example here in (R)-2-octyl tosylate we can number as OTs(1)>C6H13(2)>CH3(3)>H(4).
- Now first check the position of hydrogen. If it is placed horizontally then when going from 1 to 4 in clockwise direction as shown will be R form and if it is anticlockwise then S form. It will be vice-versa when hydrogen is placed vertically.
Now we will proceed the reaction with solvation of (R)-2-octyl tosylate.
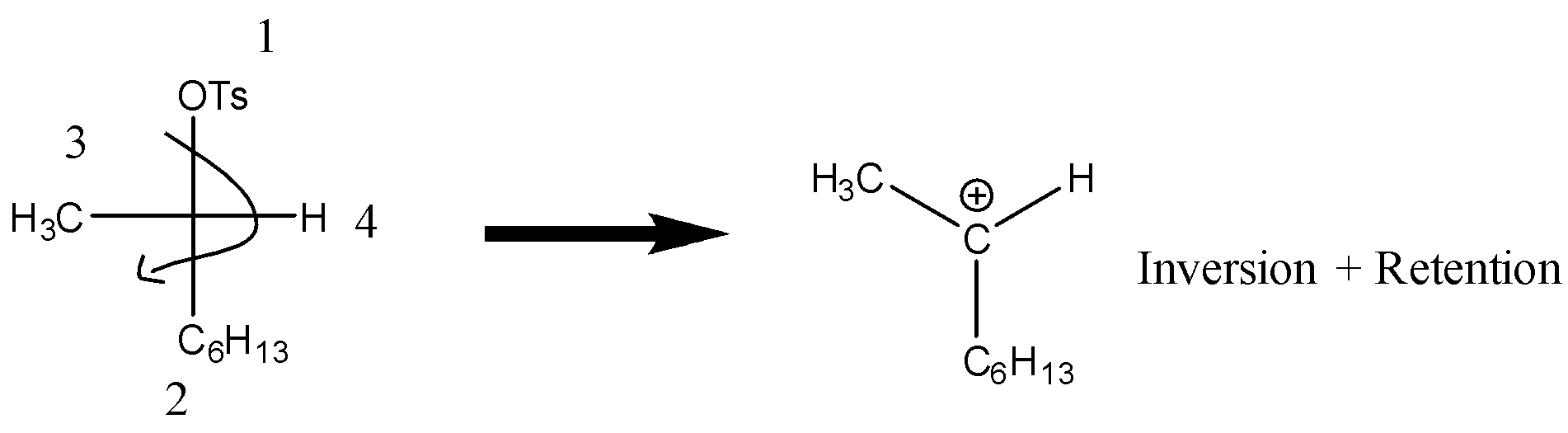
- During solvolysis tosylate will be removed and planar carbocation is formed. During this process water will act as a nucleophile and product formed may be R and S as under ideal SN1 condition there will be (inversion and Retention) both. Therefore, the product will be both R and S form. The product formed will be 2-octanol in both R and S form.
- Now we will check all options one by one. Option (A) R-2-octanol and S-2-octanol(excess). In this S form is in excess but under ideal SN1 condition both forms are formed in equal amounts.
Option (B) R-2-octanol and S-2-octanol. This is the correct option as both R and S are formed in equal amounts.
Option (C) R-2-octanol only. The product formed is only R but under ideal SN1 condition both forms are formed in equal amounts.
Option (D) S-2-octanol only. The product formed is only S but under ideal SN1 condition both forms are formed in equal amounts. The correct answer is option “B” .
Note: Under ideal SN1 condition both retention and inversion occurs. On the other hand SN2 condition only inversion occurs.
- A counter clockwise configuration is S(Sinister, left configuration) and clockwise direction is R(rectus, right configuration).
