Question
Question: (R)-\(2\)-iodobutane is treated with \(NaI\) in acetone and allow to stand for a long time .the prod...
(R)-2-iodobutane is treated with NaI in acetone and allow to stand for a long time .the product eventually formed is:
A.(R)- 2-iodobutane
B.(S)- 2- iodobutane
C.±2-iodobutane
D.(±)−1,2−iodobutane
Solution
The SN2 reaction is a nucleophilic substitution reaction, in this a bond is broken and another is shaped synchronously. Two reacting species are kept inside the rate determining step of the reaction. The term 'SN2' stands for – Substitution Nucleophilic Bimolecular.
Complete Step by step solution:
I−1 is a good nucleophile as well as a very good leaving group. Therefore, if (R)-2 -iodobutane is controlled with NaI, then it’s repeated SN2 reactions occur. As a final result, later a racemic aggregate of (±)−2− iodobutane is acquired.
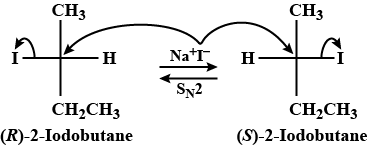
If we see the above explanation according to it, the correct answer is ±2-iodobutane .
So, the correct answer is option C.
Additional information:
The answer proceeds through a bottom attack by the nucleophile on the substrate. The nucleophile methods the given substrate at an altitude of 180 degree to the carbon-leaving institution bond or to the carbon leaving group. The carbon-nucleophile bond forms and carbon-leaving organization bond breaks simultaneously through a transition nation.
Now, the leaving organization is driven out of the transition state on the other side of the carbon-nucleophile bond, forming the specified product. It is vital to note that the product is shaped with an inversion of the tetrahedral geometry on the atom within the center.
Note: The SN2 reaction is a good example of stereospecific reactions. In this one there are in which one of a kind stereoisomers react to provide different stereoisomers of the product. Also, SN2 response is the highest common instance of Walden inversion, in which an uneven carbon atom(that is an odd number of carbon) undergoes inversion of configurations.
