Question
Question: A and B in the following reaction are ...
A and B in the following reaction are
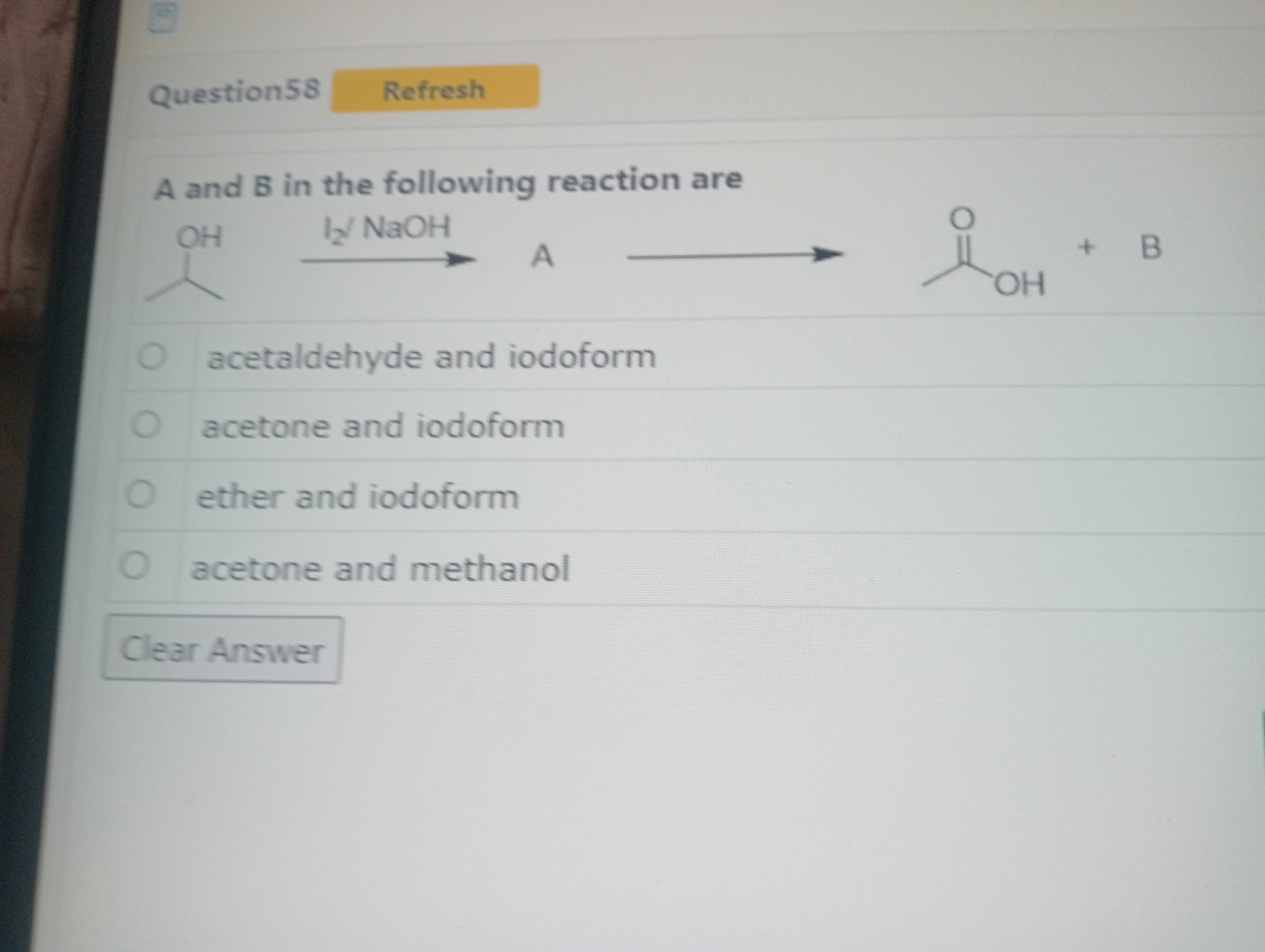
acetaldehyde and iodoform
acetone and iodoform
ether and iodoform
acetone and methanol
acetone and iodoform
Solution
The reaction shown is a classic example of the iodoform reaction (a type of haloform reaction).
The starting material is propan-2-ol (isopropyl alcohol):
This compound contains a methyl carbinol group (-CH(OH)CH₃), which is a prerequisite for a positive iodoform test.
The reaction proceeds in two main steps under the given conditions (I₂/NaOH):
Step 1: Oxidation of Propan-2-ol to Acetone (Compound A)
Alcohols containing the -CH(OH)CH₃ group are first oxidized to the corresponding methyl ketones in the presence of halogen and base. Propan-2-ol is oxidized to acetone (propanone):
CH3CH(OH)CH3I2/NaOHCH3COCH3Therefore, A is acetone.
Step 2: Haloform Reaction of Acetone to form Iodoform (Compound B) and Sodium Acetate
Acetone (Compound A), being a methyl ketone (containing the -COCH₃ group), then undergoes further reaction with I₂/NaOH. This involves complete iodination of the methyl group attached to the carbonyl, followed by cleavage of the tri-iodomethyl group.
CH3COCH3I2/NaOHCH3COONa+CHI3The products are sodium acetate (the salt of acetic acid) and iodoform. The reaction scheme shows the formation of a carboxylic acid (or its salt, given the basic conditions) which is acetic acid, and compound B. Therefore, B is iodoform (CHI₃).
The complete reaction sequence is:
CH3CH(OH)CH3I2/NaOHCH3COCH3 (A)I2/NaOHCH3COONa+CHI3 (B)Based on this analysis, A is acetone and B is iodoform.
Explanation of the solution:
Propan-2-ol undergoes oxidation to acetone (A) in the presence of I₂/NaOH. Acetone (A) then reacts further under the same conditions to yield sodium acetate and iodoform (B) via the haloform reaction.
