Question
Question: The order of rate of reaction when the following compounds will be reacted with NaOH is ...
The order of rate of reaction when the following compounds will be reacted with NaOH is
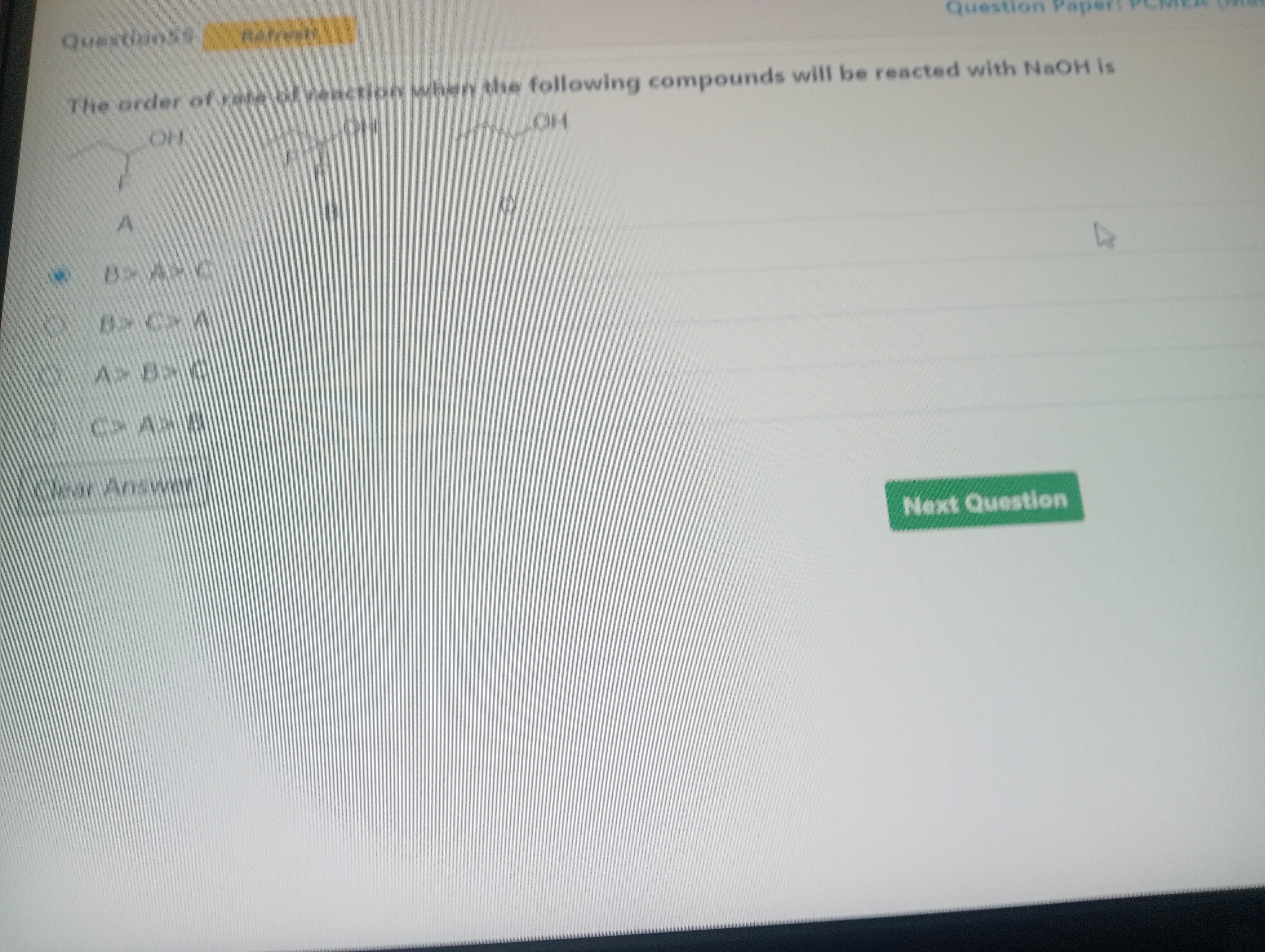
B> A> C
B> C> A
A> B> C
C> A> B
B> A> C
Solution
The reaction of the given compounds with NaOH is an acid-base reaction, where NaOH acts as a base and deprotonates the hydroxyl group of the alcohols. The rate of this reaction is directly proportional to the acidity of the alcohol. A more acidic alcohol will react faster with NaOH.
The acidity of alcohols is primarily determined by the stability of their conjugate base (alkoxide ion, R-O⁻). Electron-withdrawing groups stabilize the negative charge on the oxygen atom, thereby increasing the acidity of the alcohol. Electron-donating groups destabilize the negative charge, decreasing the acidity.
Let's analyze the given compounds:
-
Compound A (2-fluoro-1-propanol):
Structure:CH₃-CHF-CH₂-OH
It has one fluorine atom (-F) on the carbon adjacent to the carbon bearing the -OH group. Fluorine is a highly electronegative atom and exerts a strong electron-withdrawing inductive effect (-I effect). This -I effect withdraws electron density from the carbon chain, making the oxygen atom of the -OH group more electron-deficient and thus increasing the polarization of the O-H bond. This stabilizes the resulting alkoxide ion (CH₃-CHF-CH₂-O⁻), making compound A more acidic than a simple primary alcohol. -
Compound B (2,2-difluoro-1-propanol):
Structure:CH₃-CF₂-CH₂-OH
It has two fluorine atoms (-F) on the carbon adjacent to the carbon bearing the -OH group. The cumulative -I effect of two fluorine atoms is much stronger than that of a single fluorine atom. This strong electron-withdrawal significantly stabilizes the alkoxide ion (CH₃-CF₂-CH₂-O⁻), making compound B the most acidic among the three. -
Compound C (1-propanol):
Structure:CH₃-CH₂-CH₂-OH
This is a simple primary alcohol with no electron-withdrawing groups. The alkyl groups (ethyl group) are slightly electron-donating (+I effect). This electron-donating effect destabilizes the negative charge on the oxygen of the alkoxide ion (CH₃-CH₂-CH₂-O⁻), making compound C the least acidic among the three.
Order of Acidity:
Based on the inductive effects, the order of acidity is:
B (two -F groups) > A (one -F group) > C (no -F groups, alkyl groups are +I)
Therefore, Acidity: B > A > C
Order of Rate of Reaction with NaOH:
Since the rate of reaction with NaOH depends on the acidity of the alcohol, the order of reaction rates will be the same as the order of acidity.
Rate of Reaction: B > A > C
