Question
Question: Which of the following undergoes fast hydrolysis?...
Which of the following undergoes fast hydrolysis?
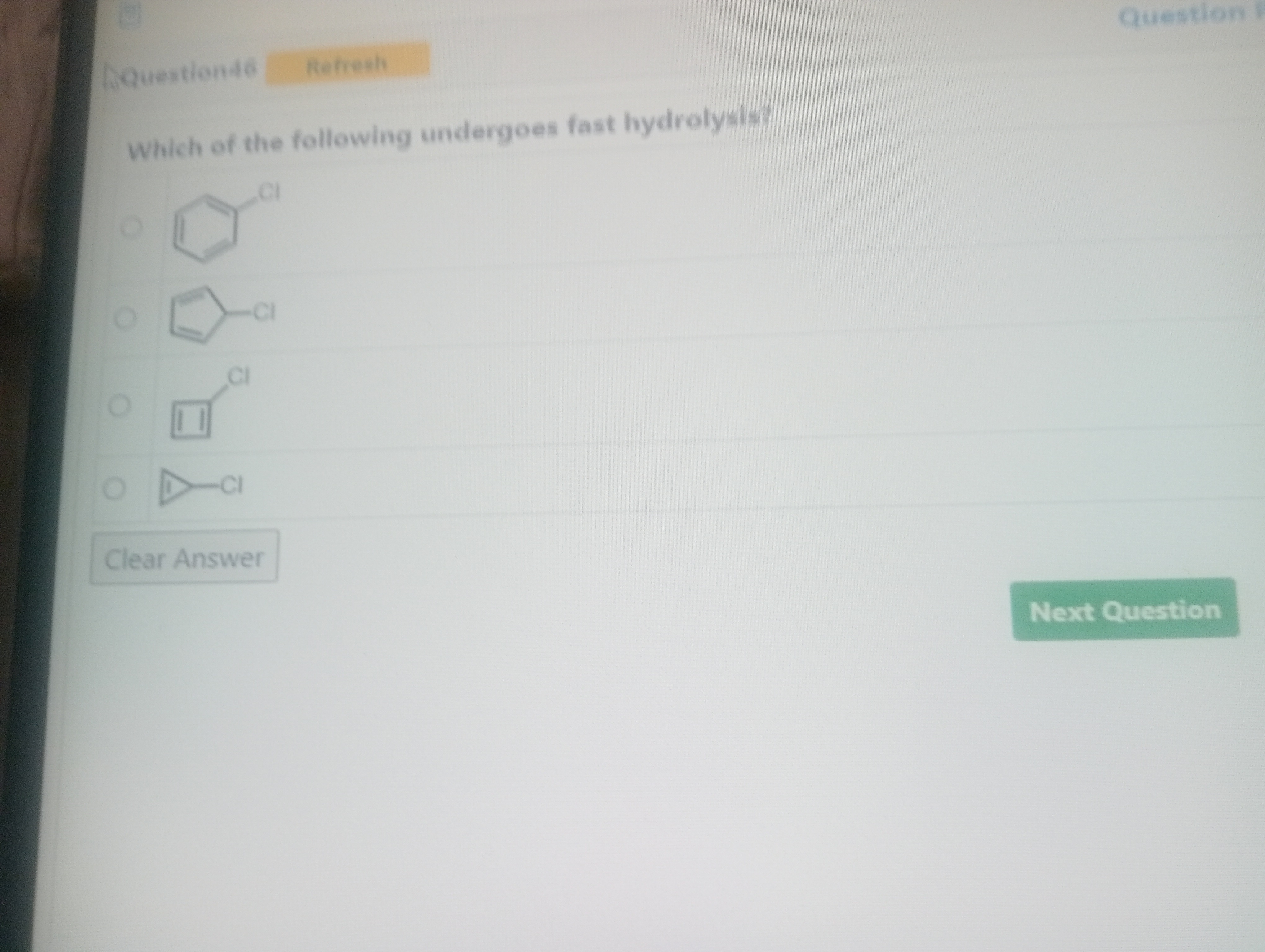
Chlorobenzene
1-Chlorocyclopentadiene
1-Chlorocyclobutene
1-Chlorocyclopropene
Option 4
Solution
Fast hydrolysis implies a favorable nucleophilic substitution reaction. For vinyl/aryl halides, this typically occurs via an SN1 mechanism if a highly stable carbocation can be formed. Among the given options, only 1-chlorocyclopropene forms a highly stable aromatic carbocation (cyclopropenyl cation, 2π electrons, obeys Hückel's rule for n=0) upon departure of the chloride ion. The other options form highly unstable (phenyl, cyclopentadienyl) or less stable (cyclobutenyl) carbocations, making their hydrolysis very slow or requiring harsh conditions.
The rate of hydrolysis for organic halides is primarily determined by the stability of the carbocation formed in the rate-determining step for SN1 reactions, or by the electrophilicity of the carbon and steric hindrance for SN2 reactions. Vinyl and aryl halides generally resist nucleophilic substitution under normal conditions due to the partial double bond character of the C-X bond (due to resonance) and the sp2 hybridization of the carbon atom bonded to the halogen. However, if the carbocation formed is exceptionally stable, SN1 hydrolysis can occur rapidly.
Let's analyze each option:
-
Chlorobenzene:
- Structure: Cl-C₆H₅
- The chlorine atom is directly attached to an sp2 hybridized carbon of the benzene ring.
- If a carbocation were to form (SN1 mechanism), it would be a phenyl cation (C₆H₅⁺). Phenyl cations are highly unstable because the positive charge resides in an sp2 orbital within the plane of the ring, making it very difficult to achieve. Therefore, chlorobenzene undergoes hydrolysis only under very harsh conditions (e.g., Dow's process).
-
1-Chlorocyclopentadiene:
- Structure: A 5-membered ring with two double bonds and a chlorine attached to an sp2 carbon of one of the double bonds.
- If a carbocation were to form, it would be a cyclopentadienyl cation. This cation has 4π electrons (from two double bonds). According to Hückel's rule (4nπ electrons), a system with 4nπ electrons is anti-aromatic and highly unstable. Thus, this compound is highly unreactive towards SN1 hydrolysis.
-
1-Chlorocyclobutene:
- Structure: A 4-membered ring with one double bond and a chlorine attached to an sp2 carbon of the double bond.
- If a carbocation were to form, it would be a cyclobutenyl cation. This cation is not aromatic and the positive charge is on an sp2 carbon, making it unstable. The ring strain also contributes to its instability.
-
1-Chlorocyclopropene:
- Structure: A 3-membered ring with one double bond and a chlorine attached to an sp2 carbon of the double bond.
- If the chlorine leaves, a cyclopropenyl cation is formed:
- The cyclopropenyl cation is a cyclic, planar system with 2π electrons. According to Hückel's rule (4n+2π electrons), for n=0, 4n+2 = 2π electrons, which makes the cyclopropenyl cation aromatic and thus highly stable.
- The formation of this highly stable aromatic carbocation significantly lowers the activation energy for the SN1 reaction, leading to very fast hydrolysis.
Conclusion: The formation of a highly stable aromatic cyclopropenyl cation makes 1-chlorocyclopropene undergo fast hydrolysis via an SN1 mechanism.
