Question
Question: Question: Two types of \(FXF\) angles are present in which of the following molecules \(\left( {X = ...
Question: Two types of FXF angles are present in which of the following molecules (X=S,Xe,C)?
A: SF4
B: XeF4
C: SF6
D: CF4
Solution
Bond angle is the angle that is formed between two bonds. For the formation of bond angle there must be at least three atoms and two bonds between these atoms. It is not necessary that bond angle is the same between all the bonds of a molecule.
Complete step by step answer:
In this question we have to attach a central atom with fluorine atoms so that the bond angle will be different for two pairs of bonds. To compare the bond angle there must be four bonds of the central atom with four fluorine atoms (as one bond angle is formed between two atoms).
CF4 has tetrahedral geometry. In this structure all the bond angles are equal. This means CF4 is not the answer as we have to find the molecule with two types of bond angles.
SF6 has octahedral geometry. In this structure all the bond angles are equal. This means SF6 is also not the answer as we have to find the molecule with two types of bond angles.
XeF4 also has tetrahedral geometry. In this structure all the bond angles are equal. This means XeF4 is also not the answer as we have to find the molecule with two types of bond angles.
SF4 has seesaw geometry. In this structure all the bond angles are not equal. There are two bond angles in this geometry. This means SF4 is the answer as we have to find the molecule with two types of bond angles.
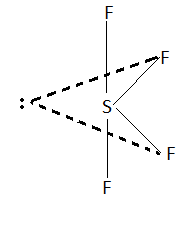
Structure of SF4
So, the correct answer is option A .
Note:
Hybridization of SF4 is sp3d. Hybridization is intermixing of atomic orbitals of slightly different energies but of the same molecule to form new hybrid orbital whose energy will be same but different from parent orbitals.
