Question
Question: Question: Select the correct statement(s) regarding defects in solids. This question has multiple ...
Question: Select the correct statement(s) regarding defects in solids.
This question has multiple correct options.
A. Frenkel defect is usually favored by a very small difference in the size of cation and anion
B. Frenkel defect is a dislocation defect.
C. Trapping of an electron in the lattice leads to the formation of F−center.
D. Schottky defects have no effect on the physical properties of solids.
Solution
In solid crystalline materials the atoms or molecules are arranged regularly and periodically in three dimensions. Unit cell is the smallest structure of crystal lattice which repeats over and over. There are some defects in crystal structure as well. Some of the defects are: frenkel defect, schottky defect, interstitial defect etc.
Complete step by step answer: As we know crystal structure is the periodic and repetitive arrangement of unit cells. Unit cell is the smallest unit of crystal structure. There are some defects in solids as well. In this question we have to mark the options that are correct regarding defects in solids.
Frenkel defect: Frenkel defect is also called dislocation defect. It is a type of point defect. In this defect an atom or smaller ion leaves its space in the lattice and becomes interstitial by lodging in a nearby location.
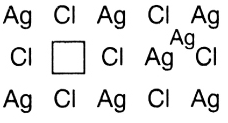
Above picture shows a frenkel defect. In this picture we can see that the silver ion leaves its space (marked as a rectangular block) and acquires some interstitial site. This is a frenkel defect. In the frenkel defect the ion which is smaller in size dislocates but there is no such condition like there should be a small difference in size of cation and anion. This means option A is incorrect and option B is correct as stated above. The frenkel defect is a dislocation defect because ion left its space and acquired some other space.
In schottky defects also ions leave their space but those ions do not acquire some other space in lattice. In schottky defect ions leave their space permanently such that the overall charge on lattice is zero. This means in schottky defects equal numbers of cations and anions are missing. Density is physical quantity. Due to schottky defect mass of lattice will decrease as ions leave their space but there is no change in volume of the unit cell. This means density of compound will decrease (because density is directly proportional to mass). Option D states that there will be no change in physical property due to schottky defect which is incorrect because density is changing.
F−center is a type of crystallographic defect in which an anionic vacancy in a crystal is occupied by one or more electrons. In this defect anion leaves its space which is then occupied by an electron. This electron tends to gain light in the visible region of the spectrum due to which color is imparted to the compound. According to option C trapping of electrons leads to formation of F−center which is correct.
So, the correct answers are “Option B and C”.
Note: Schottky defect occurs when there is a very small difference in size of cation and anions. Option A would be correct if schottky defect would be written in place of frenkel defect. This statement is correct for schottky defects.
