Question
Question: Calculate the Ksp of AgI with the help of following cell $Ag_{(s)}|AgI_{(s)}|I_{(aq)}^{-}(0.05M)||A...
Calculate the Ksp of AgI with the help of following cell
Ag(s)∣AgI(s)∣I(aq)−(0.05M)∣∣Ag(aq)+(0.05M)∣Ag(s);Ecell=0.7884V
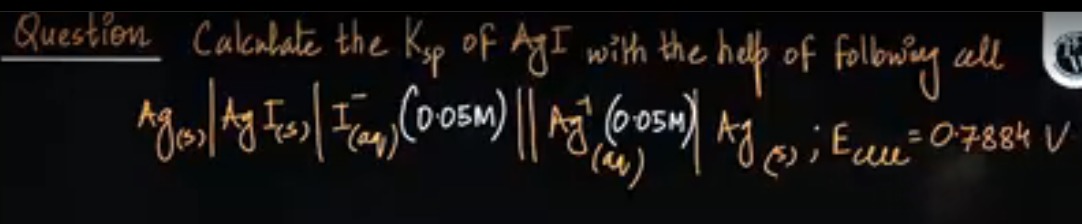
Answer
Ksp=1.1×10−16
Explanation
Solution
The given cell's potential (Ecell) is related to the standard potentials of the half-reactions and the concentrations of the ions by the Nernst equation. The standard cell potential (Ecello) for this cell, which involves the formation of AgI from its ions, is directly related to the solubility product (Ksp) of AgI. By substituting the given concentrations and measured cell potential into the derived Nernst equation, the Ksp value for AgI can be calculated.
