Question
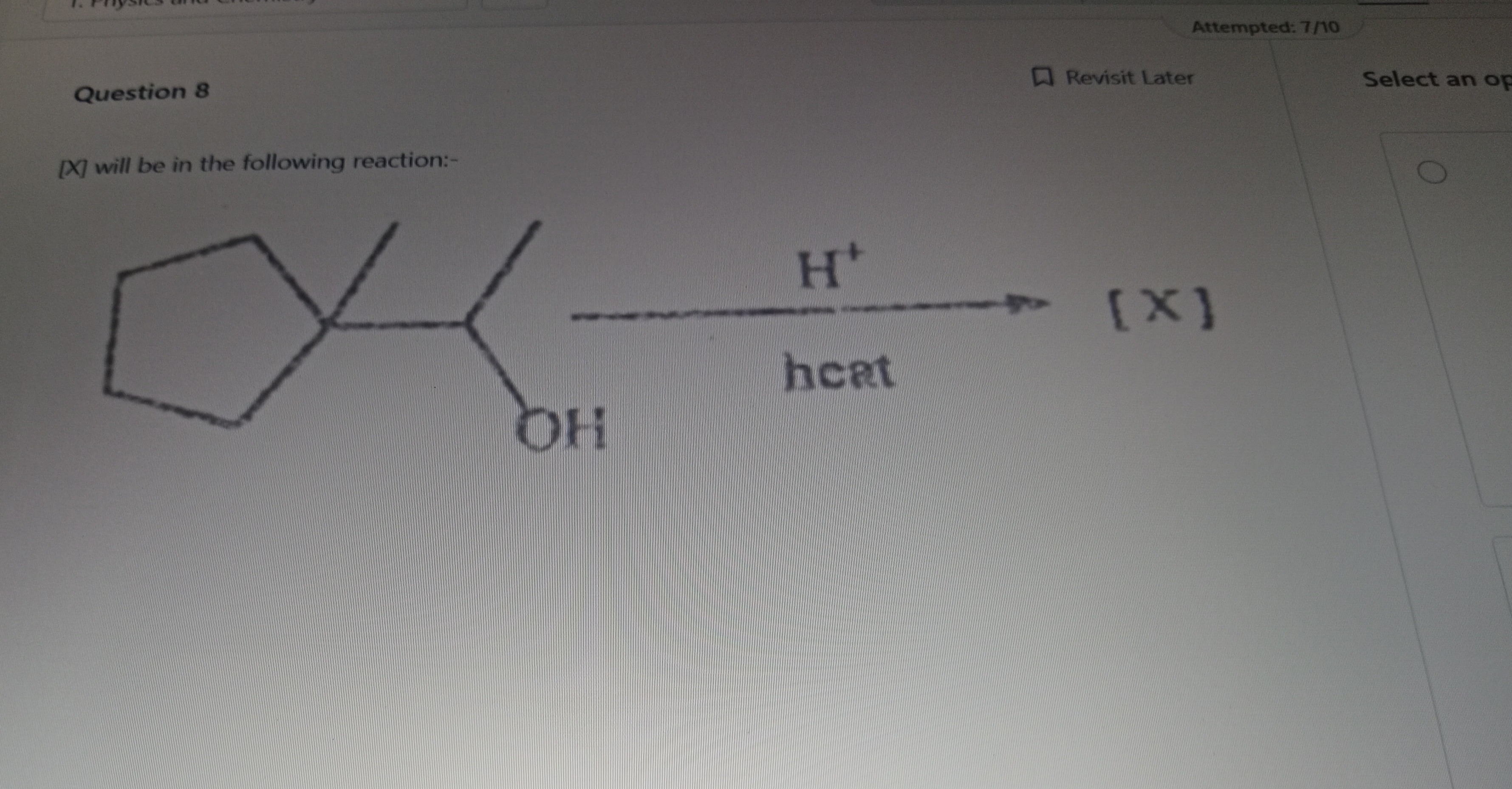
Question: [X] will be in the following reaction:- $H^+$ [X] heat OH ...
[X] will be in the following reaction:-
H+ [X] heat OH
Answer
1-methylcyclohex-1-ene
Explanation
Solution
The reaction is an acid-catalyzed dehydration of an alcohol. The alcohol first protonates, then loses water to form a secondary carbocation. This carbocation undergoes ring expansion from a 5-membered to a 6-membered ring to relieve strain and then a 1,2-hydride shift to form a more stable tertiary carbocation. Finally, deprotonation of the tertiary carbocation yields the most stable (most substituted) alkene product, which is 1-methylcyclohex-1-ene.
