Question
Question: Which is correct...
Which is correct
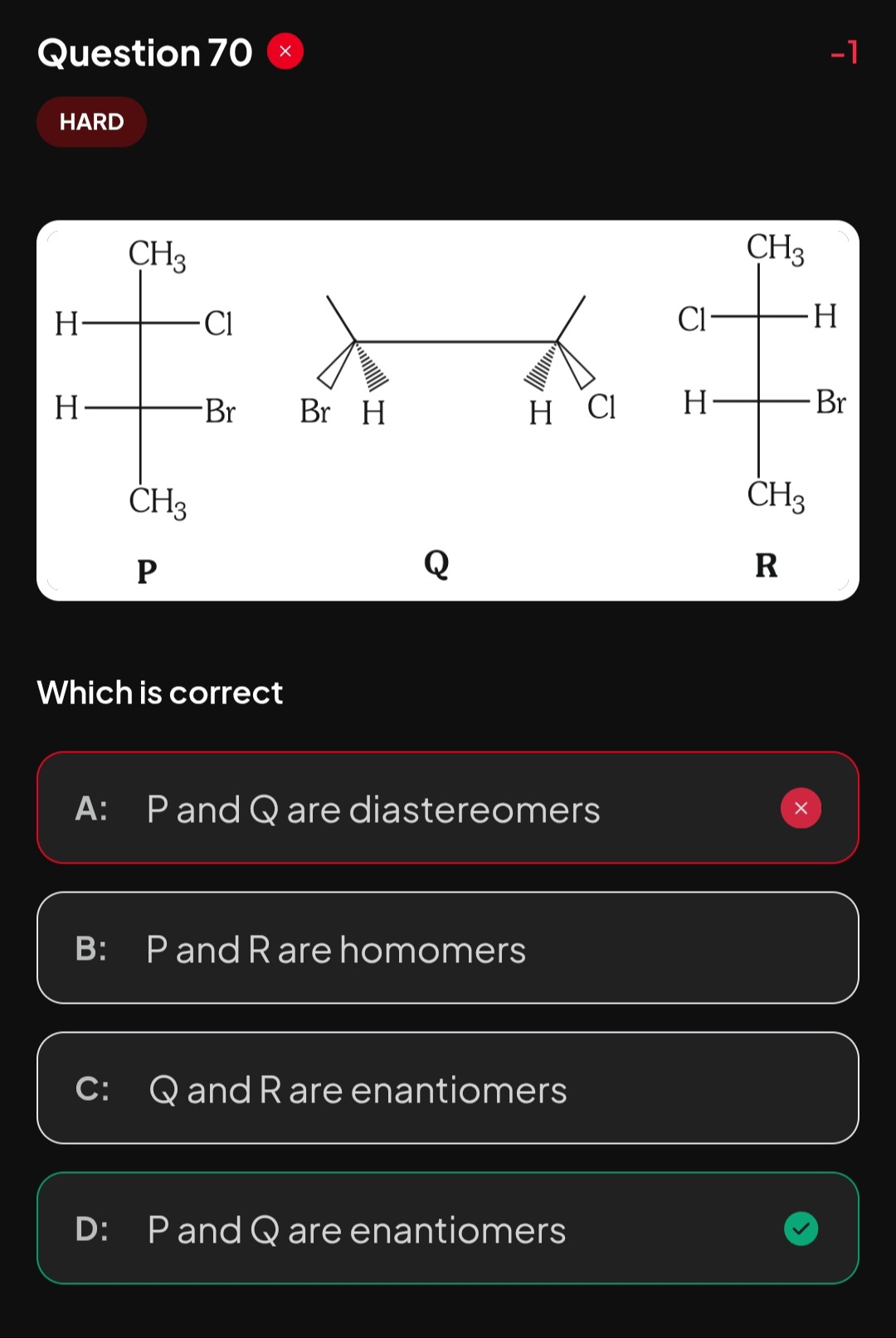
P and Q are diastereomers
P and R are homomers
Q and R are enantiomers
P and Q are enantiomers
P and Q are diastereomers
Solution
The problem requires us to determine the relationship between three given stereoisomers P, Q, and R. All three structures represent 2-bromo-3-chlorobutane, which has two chiral centers at C2 and C3. To establish the relationship, we will assign the R/S configuration to each chiral center in all three structures.
1. Assigning R/S Configuration for Structure P: Structure P is given as a Fischer projection.
CH3
|
H-C2-Cl
|
H-C3-Br
|
CH3
-
For C2 (top chiral carbon):
- Priorities of groups attached to C2: Cl (1), -CH(Br)CH3 (C3 carbon, 2), -CH3 (3), H (4).
- H is on a horizontal line (coming out).
- Path 1 -> 2 -> 3 (Cl -> C3 -> CH3) is clockwise (R).
- Since H is on a horizontal line, the actual configuration is the opposite.
- Therefore, C2 is (S).
-
For C3 (bottom chiral carbon):
- Priorities of groups attached to C3: Br (1), -CH(Cl)CH3 (C2 carbon, 2), -CH3 (3), H (4).
- H is on a horizontal line (coming out).
- Path 1 -> 2 -> 3 (Br -> C2 -> CH3) is clockwise (R).
- Since H is on a horizontal line, the actual configuration is the opposite.
- Therefore, C3 is (S).
So, Structure P is (2S, 3S)-2-bromo-3-chlorobutane.
2. Assigning R/S Configuration for Structure R: Structure R is also given as a Fischer projection.
CH3
|
Cl-C2-H
|
H-C3-Br
|
CH3
-
For C2 (top chiral carbon):
- Priorities of groups attached to C2: Cl (1), -CH(Br)CH3 (C3 carbon, 2), -CH3 (3), H (4).
- H is on a horizontal line (coming out).
- Path 1 -> 2 -> 3 (Cl -> C3 -> CH3) is counter-clockwise (S).
- Since H is on a horizontal line, the actual configuration is the opposite.
- Therefore, C2 is (R).
-
For C3 (bottom chiral carbon):
- Priorities of groups attached to C3: Br (1), -CH(Cl)CH3 (C2 carbon, 2), -CH3 (3), H (4).
- H is on a horizontal line (coming out).
- Path 1 -> 2 -> 3 (Br -> C2 -> CH3) is counter-clockwise (S).
- Since H is on a horizontal line, the actual configuration is the opposite.
- Therefore, C3 is (R).
So, Structure R is (2R, 3R)-2-bromo-3-chlorobutane.
3. Assigning R/S Configuration for Structure Q: Structure Q is given as a Newman projection with wedge/dash bonds.
To convert this Newman projection to a Fischer projection:
- Keep the CH3 groups vertical (anti-periplanar, as shown).
- For the front carbon (C2): Br is left and wedge (coming out), H is right and dash (going in). In Fischer, horizontal lines are coming out. So, Br is on the left, H is on the right.
- For the back carbon (C3): Cl is right and wedge (coming out), H is left and dash (going in). So, Cl is on the right, H is on the left.
The Fischer projection for Q is:
CH3
|
Br-C2-H
|
H-C3-Cl
|
CH3
-
For C2 (top chiral carbon):
- Priorities: Br (1), -CH(Cl)CH3 (C3 carbon, 2), -CH3 (3), H (4).
- H is on a horizontal line (coming out).
- Path 1 -> 2 -> 3 (Br -> C3 -> CH3) is counter-clockwise (S).
- Since H is on a horizontal line, the actual configuration is the opposite.
- Therefore, C2 is (R).
-
For C3 (bottom chiral carbon):
- Priorities: Cl (1), -CH(Br)CH3 (C2 carbon, 2), -CH3 (3), H (4).
- H is on a horizontal line (coming out).
- Path 1 -> 2 -> 3 (Cl -> C2 -> CH3) is clockwise (R).
- Since H is on a horizontal line, the actual configuration is the opposite.
- Therefore, C3 is (S).
So, Structure Q is (2R, 3S)-2-bromo-3-chlorobutane.
Summary of Configurations:
- P: (2S, 3S)
- Q: (2R, 3S)
- R: (2R, 3R)
4. Evaluating the Options:
-
A: P and Q are diastereomers
- P: (2S, 3S)
- Q: (2R, 3S)
- They differ in configuration at one chiral center (C2: S vs R) and are the same at the other (C3: S vs S). Stereoisomers that are not mirror images and differ at at least one chiral center but not all are diastereomers. So, A is correct.
-
B: P and R are homomers
- P: (2S, 3S)
- R: (2R, 3R)
- Homomers are identical molecules. P and R have opposite configurations at both chiral centers. They are non-superimposable mirror images. Thus, P and R are enantiomers, not homomers. So, B is incorrect.
-
C: Q and R are enantiomers
- Q: (2R, 3S)
- R: (2R, 3R)
- They are the same at one chiral center (C2: R vs R) and opposite at the other (C3: S vs R). Thus, Q and R are diastereomers, not enantiomers. So, C is incorrect.
-
D: P and Q are enantiomers
- P: (2S, 3S)
- Q: (2R, 3S)
- They are opposite at one chiral center (C2: S vs R) and the same at the other (C3: S vs S). Thus, P and Q are diastereomers, not enantiomers. So, D is incorrect.
Based on the detailed R/S analysis, option A is the correct statement.
