Question
Question: For the given reversible reaction $2A + B \rightleftharpoons 3C + D$ The expression for the formati...
For the given reversible reaction 2A+B⇌3C+D
The expression for the formation of C is given as: +dtdC=2.4×10−4[A]2[B]−6×10−5[C]3[D]
The value of equilibrium constant for the given reaction is (unit of Keq is mol/L for all)
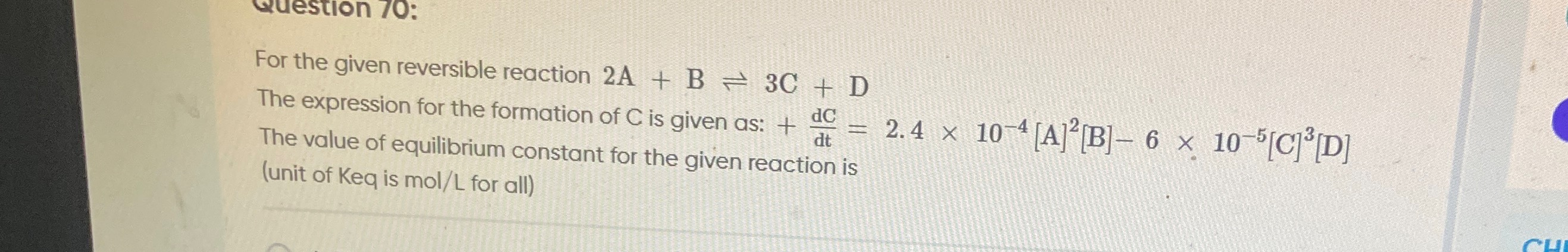
4
Solution
The given reversible reaction is: 2A+B⇌3C+D
The expression for the rate of formation of C is given as: +dtdC=2.4×10−4[A]2[B]−6×10−5[C]3[D]
At equilibrium, the net rate of reaction is zero, which means the rate of formation of C is zero: dtdC=0
Therefore, at equilibrium: 2.4×10−4[A]2[B]−6×10−5[C]3[D]=0
Rearranging the equation, we get: 2.4×10−4[A]2[B]=6×10−5[C]3[D]
The equilibrium constant (Keq) for the given reaction is defined as the ratio of the product concentrations to the reactant concentrations, each raised to the power of their stoichiometric coefficients: Keq=[A]2[B][C]3[D]
From the equilibrium condition derived above, we can express Keq: [A]2[B][C]3[D]=6×10−52.4×10−4
Now, calculate the value of Keq: Keq=6×10−52.4×10−4 Keq=62.4×10−510−4 Keq=0.4×10(−4−(−5)) Keq=0.4×10(−4+5) Keq=0.4×101 Keq=4
The unit of Keq for this reaction: Δn=(3+1)−(2+1)=4−3=1. So, the unit is (mol/L)1=mol/L, which is consistent with the information given in the question.
