Question
Question: At room temperature, sodium crystallizes in a body centred cubic lattice with unit cell length 4.24 ...
At room temperature, sodium crystallizes in a body centred cubic lattice with unit cell length 4.24 A˚.
Theoretical density of sodium will be (Na=23):-
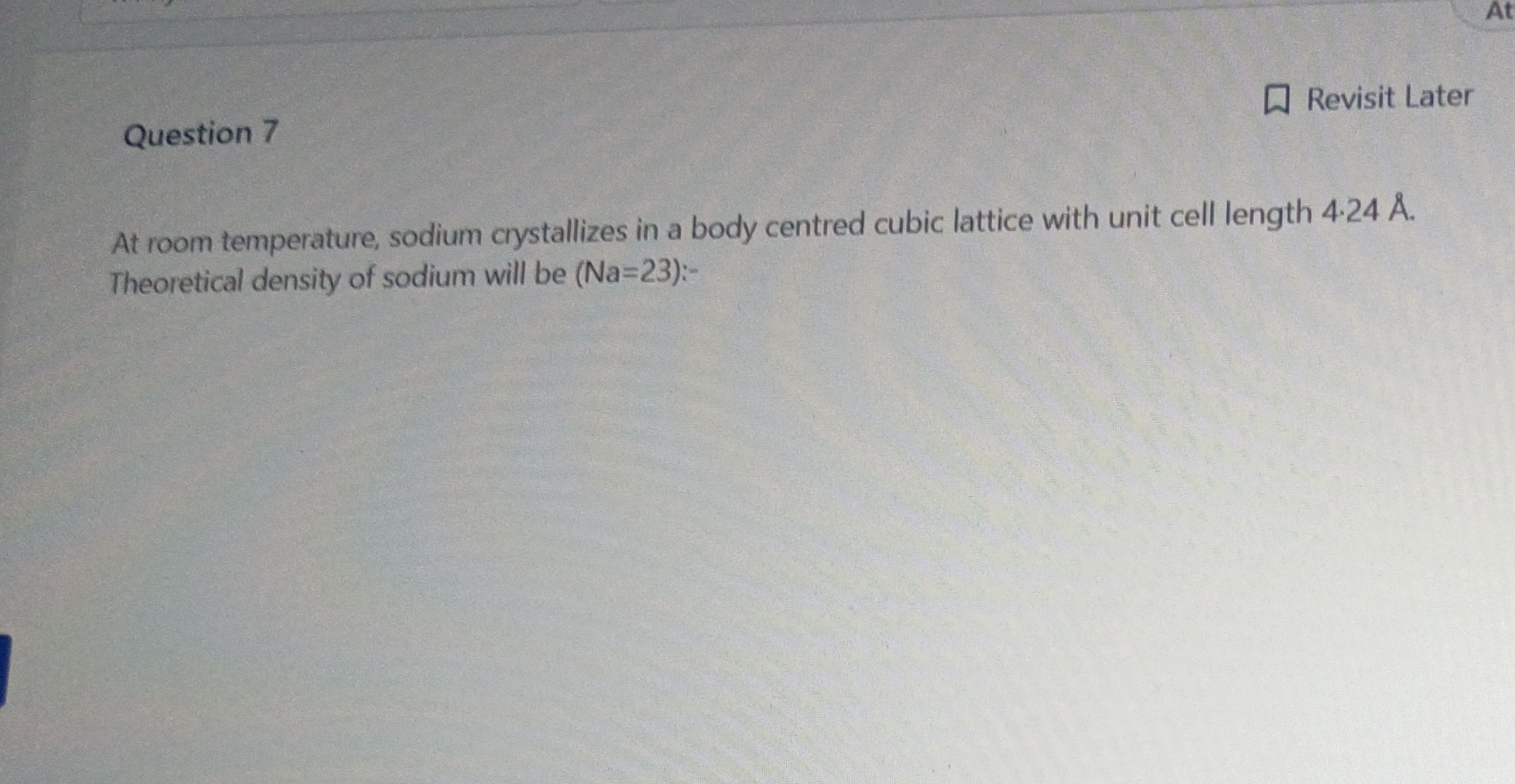
- 002 g/cm3
- 004 g/cm3
- 006 g/cm3
- 008 g/cm3
1.002 g/cm3
Solution
To calculate the theoretical density of sodium, which crystallizes in a body-centred cubic (BCC) lattice, we use the formula for the density of a unit cell:
ρ=NA×a3Z×M
Where:
- ρ is the density of the unit cell.
- Z is the number of atoms per unit cell.
- M is the molar mass of the element.
- NA is Avogadro's number (6.022×1023 mol−1).
- a is the unit cell edge length.
Given values:
- Lattice type: Body-centred cubic (BCC)
- Unit cell length (a) = 4.24 Å
- Atomic mass of Na (M) = 23 g/mol
Step 1: Determine the number of atoms per unit cell (Z) for a BCC lattice.
In a BCC lattice, there are 8 atoms at the corners and 1 atom at the body centre.
- Contribution from corner atoms: 8×81=1 atom
- Contribution from body-centred atom: 1×1=1 atom
Total number of atoms per unit cell (Z) = 1+1=2.
Step 2: Convert the unit cell length from Å to cm.
Given a=4.24A˚. Since 1A˚=10−8cm, a=4.24×10−8cm.
Step 3: Calculate the volume of the unit cell (a3).
a3=(4.24×10−8cm)3 a3=(4.24)3×(10−8)3cm3 a3=76.228544×10−24cm3
Step 4: Substitute the values into the density formula and calculate.
ρ=NA×a3Z×M ρ=6.022×1023mol−1×76.228544×10−24cm32×23g/mol ρ=6.022×76.228544×10(23−24)cm346g ρ=6.022×76.228544×10−1cm346g ρ=458.9825×10−1cm346g ρ=45.89825cm346g ρ≈1.0022g/cm3
Rounding to three significant figures, the theoretical density of sodium is 1.00g/cm3. If we keep more precision, it is 1.002g/cm3.
The density of a crystal lattice is determined by the mass of atoms within a unit cell and the volume of that unit cell. For a BCC lattice, there are 2 atoms per unit cell. The given unit cell length is converted to cm, and then its cube gives the volume. Using Avogadro's number and the atomic mass, the density is calculated using the formula ρ=NA×a3Z×M.
