Question
Question: Find the ratio of magnetic moment of electron in $He^+$ ion in ground state to the magnetic moment o...
Find the ratio of magnetic moment of electron in He+ ion in ground state to the magnetic moment of electron in H atom in 1st excited state.
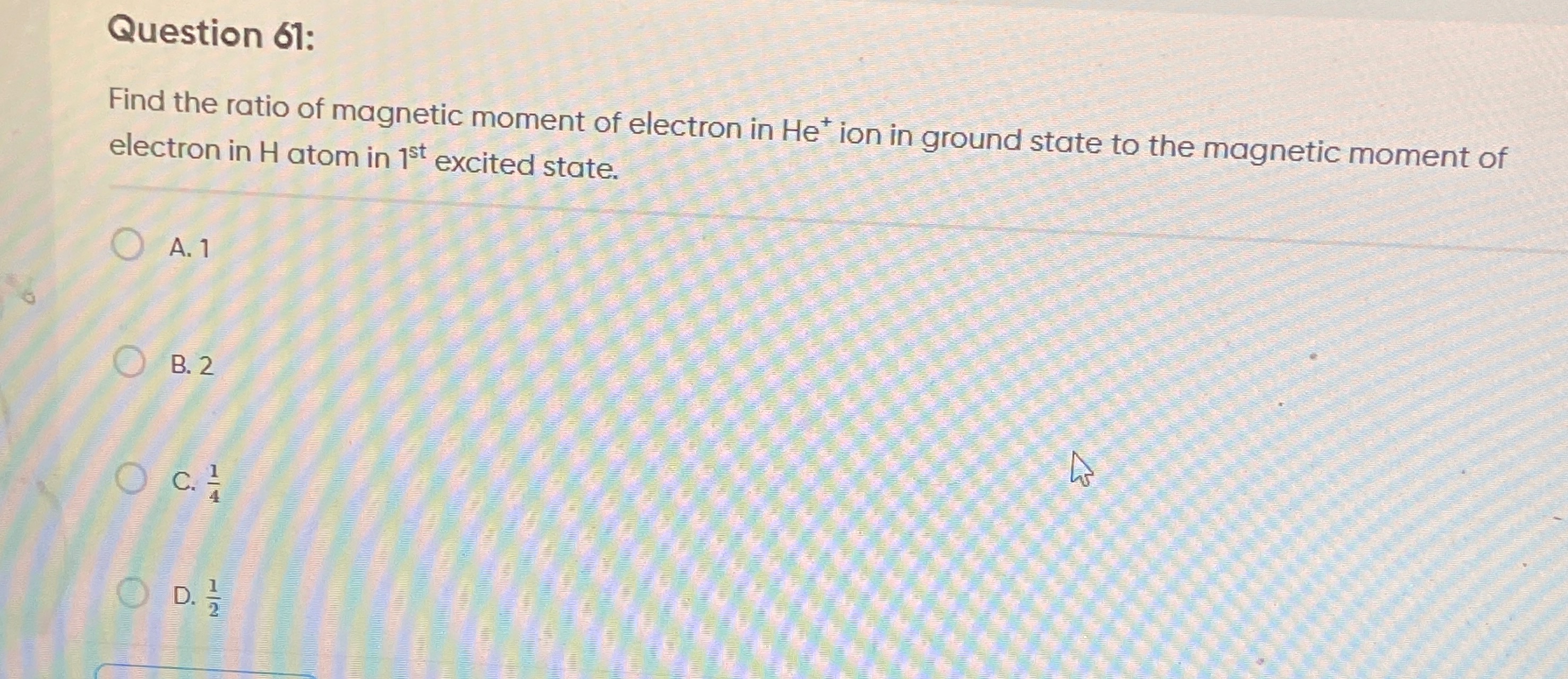
1
2
41
21
21
Solution
The magnetic moment (μ) of an electron in an orbit is given by μ=2meL, where e is the electron's charge, L is its orbital angular momentum, and m is its mass. According to Bohr's model, the orbital angular momentum is quantized as Ln=nℏ, where n is the principal quantum number and ℏ is the reduced Planck constant. Substituting Ln into the magnetic moment formula, we get μn=2me(nℏ)=n(2meℏ). The term 2meℏ is the Bohr magneton (μB), so μn=nμB. This shows that the magnetic moment is directly proportional to the principal quantum number n.
For the He+ ion in the ground state (n=1): μHe+=1×μB=μB.
For the H atom in the 1st excited state (n=2): μH=2×μB=2μB.
The ratio is: Ratio=μHμHe+=2μBμB=21
