Question
Question: Consider a parallel reaction $k_1 = ln 81 hr^{-1}m, k_2 = ln27 hr^{-1}$. Find the time (in minutes)...
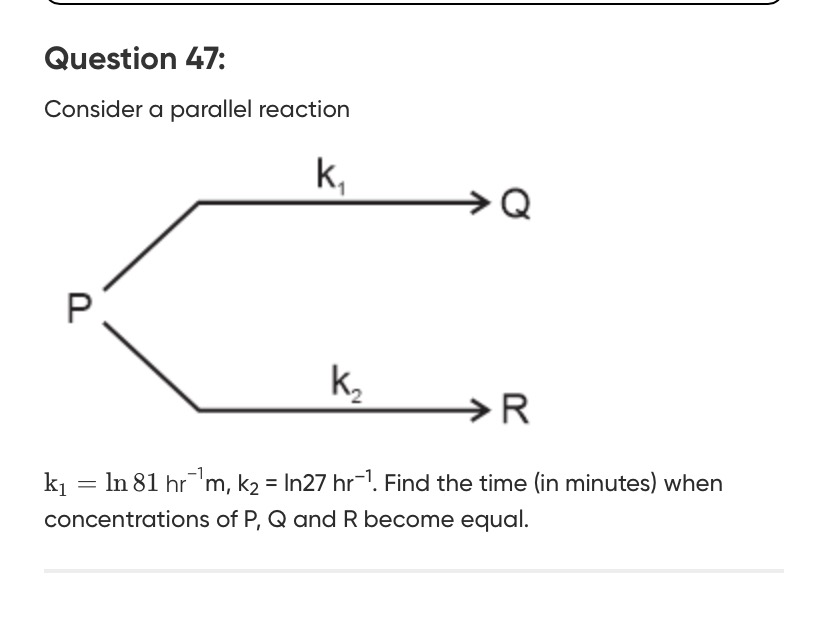
Consider a parallel reaction
k1=ln81hr−1m,k2=ln27hr−1. Find the time (in minutes) when concentrations of P, Q and R become equal.
5.407 minutes
Solution
The given reaction is a parallel first-order reaction:
Pk1Q
Pk2R
The rate constants are given as:
k1=ln81 hr−1
k2=ln27 hr−1
Let's simplify the rate constants:
k1=ln(34)=4ln3 hr−1
k2=ln(33)=3ln3 hr−1
Let [P]0 be the initial concentration of P. We assume the initial concentrations of products Q and R are zero.
For a first-order parallel reaction, the concentration of the reactant P at time t is given by:
[P]t=[P]0e−(k1+k2)t
The concentrations of the products Q and R at time t are given by:
[Q]t=k1+k2k1[P]0(1−e−(k1+k2)t)
[R]t=k1+k2k2[P]0(1−e−(k1+k2)t)
We are asked to find the time when concentrations of P, Q, and R become equal, i.e., [P]t=[Q]t=[R]t.
However, for [Q]t=[R]t to be true for t>0, it must be that k1=k2.
Since k1=4ln3 and k2=3ln3, we have k1=k2.
Therefore, [Q]t can never be equal to [R]t for t>0. This implies that the condition [P]t=[Q]t=[R]t cannot be satisfied.
In such ambiguous problems in competitive exams, where the literal interpretation is impossible, a common alternative interpretation is that the concentration of the reactant P becomes equal to the total concentration of the products (Q and R).
This means we are looking for the time when [P]t=[Q]t+[R]t.
From the conservation of mass, the total concentration remains constant:
[P]0=[P]t+[Q]t+[R]t
If we substitute the condition [P]t=[Q]t+[R]t into the conservation equation:
[P]0=[P]t+[P]t
[P]0=2[P]t
So, [P]t=2[P]0
Now, we need to find the time t when the concentration of P becomes half of its initial concentration.
Using the equation for [P]t:
2[P]0=[P]0e−(k1+k2)t
21=e−(k1+k2)t
Taking the natural logarithm on both sides:
ln(21)=−(k1+k2)t
−ln2=−(k1+k2)t
t=k1+k2ln2
First, calculate the sum of the rate constants:
k1+k2=4ln3+3ln3=7ln3 hr−1
Now, substitute this value into the equation for t:
t=7ln3ln2 hours
The question asks for the time in minutes. To convert hours to minutes, multiply by 60:
tminutes=7ln3ln2×60 minutes
Using approximate values for natural logarithms (ln2≈0.693 and ln3≈1.0986):
tminutes=7×1.09860.693×60
tminutes=7.69020.693×60
tminutes≈0.090119×60
tminutes≈5.407 minutes
The 'm' in k1=ln81hr−1m is considered a typo, as rate constants for first-order reactions have units of time−1.
