Question
Question: Match the plots of certain chemical reactions given in List-I with their characteristics given in Li...
Match the plots of certain chemical reactions given in List-I with their characteristics given in List-II and choose the correct options
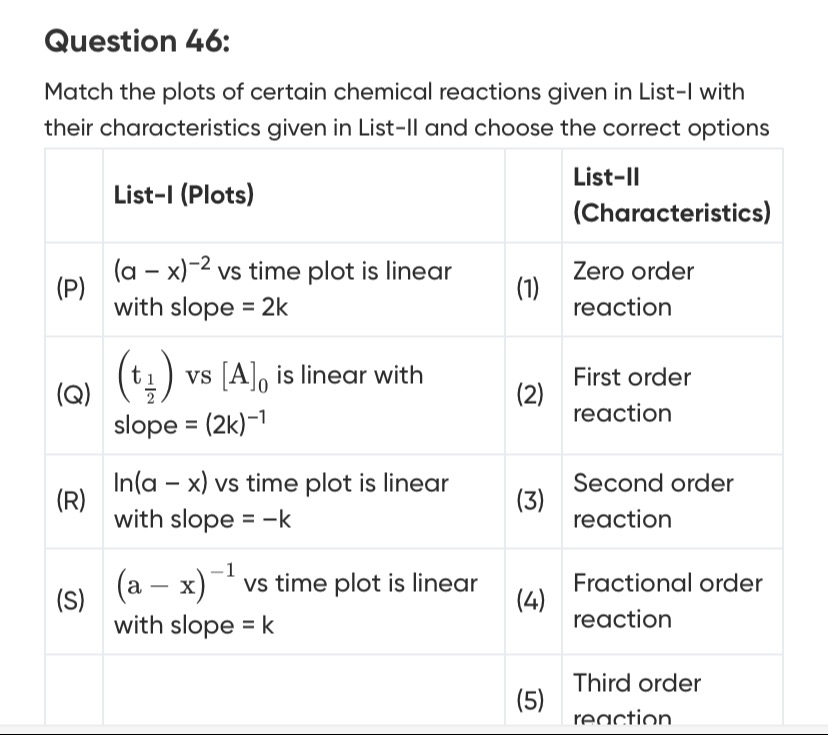
| List-I (Plots) | List-II (Characteristics) | ||
|---|---|---|---|
| (P) | (a−x)−2 vs time plot is linear with slope = 2k | (1) | Zero order reaction |
| (Q) | (2t1) vs [A]0 is linear with slope = (2k)−1 | (2) | First order reaction |
| (R) | ln(a−x) vs time plot is linear with slope = -k | (3) | Second order reaction |
| (S) | (a−x)−1 vs time plot is linear with slope = k | (4) | Fractional order reaction |
| (5) | Third order reaction |
(P) - (1)
(Q) - (2)
(R) - (3)
(S) - (4)
(P) - (5), (Q) - (1), (R) - (2), (S) - (3)
P-5, Q-1, R-2, S-3
Solution
The problem requires matching plots of chemical reactions from List-I with their corresponding characteristics (order of reaction) from List-II. We will analyze the integrated rate laws and half-life expressions for different orders of reactions.
Let [A]0 (or a) be the initial concentration and [A]t (or a−x) be the concentration at time t.
1. Zero Order Reaction:
- Integrated Rate Law: [A]t=[A]0−kt or x=kt.
- A plot of [A]t vs time is linear with slope −k.
- Half-life (t1/2): t1/2=2k[A]0.
- A plot of t1/2 vs [A]0 is linear with slope 2k1.
2. First Order Reaction:
- Integrated Rate Law: ln[A]t=ln[A]0−kt or ln(a−x)=lna−kt.
- A plot of ln[A]t (or ln(a−x)) vs time is linear with slope −k.
- Half-life (t1/2): t1/2=kln2=k0.693. (Independent of initial concentration)
3. Second Order Reaction:
- Integrated Rate Law: [A]t1=[A]01+kt or (a−x)1=a1+kt.
- A plot of [A]t1 (or (a−x)1) vs time is linear with slope k.
- Half-life (t1/2): t1/2=k[A]01.
4. Third Order Reaction (for 3A→Products):
- Integrated Rate Law: 2[A]t21−2[A]021=kt or 2(a−x)21−2a21=kt.
- Rearranging, (a−x)21=a21+2kt.
- A plot of (a−x)−2 vs time is linear with slope 2k.
Now let's match the given plots from List-I with their characteristics from List-II:
-
(P) (a−x)−2 vs time plot is linear with slope = 2k: This corresponds to the integrated rate law for a Third order reaction. Therefore, (P) matches with (5).
-
(Q) (2t1) vs [A]0 is linear with slope = (2k)−1: This describes the half-life dependence on initial concentration for a Zero order reaction. Therefore, (Q) matches with (1).
-
(R) ln(a−x) vs time plot is linear with slope = -k: This is the integrated rate law for a First order reaction. Therefore, (R) matches with (2).
-
(S) (a−x)−1 vs time plot is linear with slope = k: This is the integrated rate law for a Second order reaction. Therefore, (S) matches with (3).
Final Matching:
- (P) - (5)
- (Q) - (1)
- (R) - (2)
- (S) - (3)
The question asks to choose the correct options. Since it's a matching question, the correct options are the pairs derived above.
The final answer is P−5,Q−1,R−2,S−3
Explanation of the solution: The solution involves identifying the order of reaction based on the linearity of specific plots derived from integrated rate laws and half-life expressions.
- P: The plot of (a−x)−2 versus time being linear with slope 2k is characteristic of a third-order reaction.
- Q: For a zero-order reaction, the half-life (t1/2) is directly proportional to the initial concentration ([A]0), specifically t1/2=2k[A]0. Thus, a plot of t1/2 vs [A]0 is linear with slope 2k1.
- R: The integrated rate law for a first-order reaction is ln(a−x)=lna−kt. Therefore, a plot of ln(a−x) vs time is linear with a slope of −k.
- S: The integrated rate law for a second-order reaction (of type A→Products) is (a−x)1=a1+kt. Thus, a plot of (a−x)−1 vs time is linear with a slope of k.
