Question
Question: The rate constants of two parallel first order reactions (I) and (II) are 1.50 $\times$ 10$^{-2}$s$^...
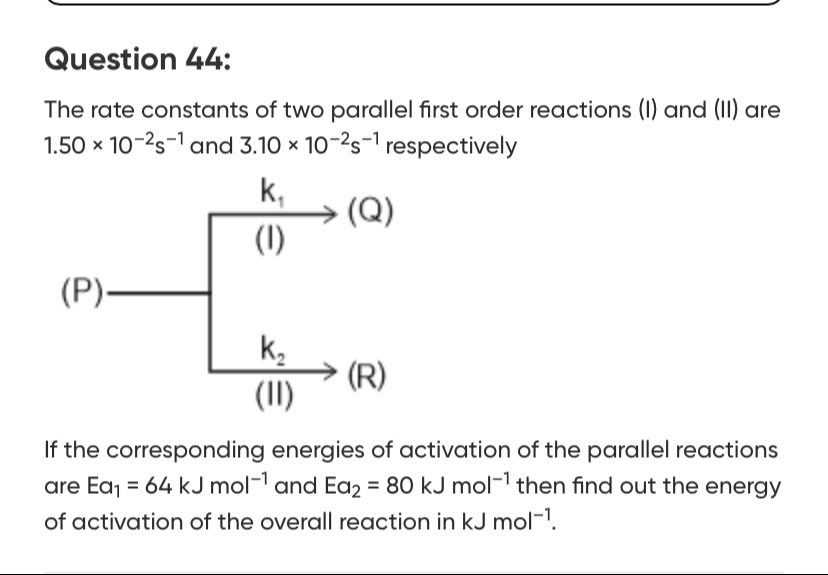
The rate constants of two parallel first order reactions (I) and (II) are 1.50 × 10−2s−1 and 3.10 × 10−2s−1 respectively
If the corresponding energies of activation of the parallel reactions are Ea1 = 64 kJ mol−1 and Ea2 = 80 kJ mol−1 then find out the energy of activation of the overall reaction in kJ mol−1.
74.78 kJ mol−1
Solution
The problem involves two parallel first-order reactions. For such reactions, the overall rate constant (koverall) is the sum of the individual rate constants (k1 and k2).
koverall=k1+k2
The Arrhenius equation relates the rate constant (k) to the activation energy (Ea) and temperature (T):
k=Ae−Ea/RT
where A is the pre-exponential factor and R is the gas constant.
To find the overall activation energy (Ea,overall), we can differentiate the Arrhenius equation with respect to temperature. Taking the natural logarithm of the Arrhenius equation:
lnk=lnA−RTEa
Differentiating with respect to temperature T:
dTd(lnk)=0−REa(−T21)=RT2Ea
Also, we know that dTd(lnk)=k1dTdk. So, k1dTdk=RT2Ea, which implies dTdk=kRT2Ea.
For the overall reaction, we have:
dTdkoverall=dTdk1+dTdk2
Substitute the expression for dTdk for each term:
koverallRT2Ea,overall=k1RT2Ea1+k2RT2Ea2
Multiplying both sides by RT2:
koverallEa,overall=k1Ea1+k2Ea2
Now, substitute koverall=k1+k2:
(k1+k2)Ea,overall=k1Ea1+k2Ea2
Therefore, the overall activation energy is:
Ea,overall=k1+k2k1Ea1+k2Ea2
Given values:
k1=1.50×10−2 s−1 Ea1=64 kJ mol−1 k2=3.10×10−2 s−1 Ea2=80 kJ mol−1
Substitute these values into the formula:
Ea,overall=1.50×10−2 s−1+3.10×10−2 s−1(1.50×10−2 s−1)(64 kJ mol−1)+(3.10×10−2 s−1)(80 kJ mol−1)
We can factor out 10−2 from the numerator and denominator:
Ea,overall=10−2(1.50+3.10)10−2(1.50×64+3.10×80) Ea,overall=1.50+3.101.50×64+3.10×80
Calculate the terms: Numerator: 1.50×64=96.0 3.10×80=248.0 Sum of numerator terms =96.0+248.0=344.0
Denominator: 1.50+3.10=4.60
Now, calculate Ea,overall:
Ea,overall=4.60344.0 Ea,overall=463440 Ea,overall=231720 Ea,overall≈74.7826 kJ mol−1
Rounding to two decimal places, the energy of activation of the overall reaction is 74.78 kJ mol−1.
Explanation of the solution: For parallel first-order reactions, the overall rate constant (koverall) is the sum of the individual rate constants (k1+k2). The relationship between the overall activation energy (Ea,overall) and individual activation energies (Ea1,Ea2) is derived by differentiating the Arrhenius equation for the overall reaction with respect to temperature. This yields the formula:
Ea,overall=k1+k2k1Ea1+k2Ea2
Substitute the given values for k1, Ea1, k2, and Ea2 into this formula and compute the result.
