Question
Question: Which of the following statements is/are correct about the reaction between Cu metal and dil. HNO3?...
Which of the following statements is/are correct about the reaction between Cu metal and dil. HNO3?
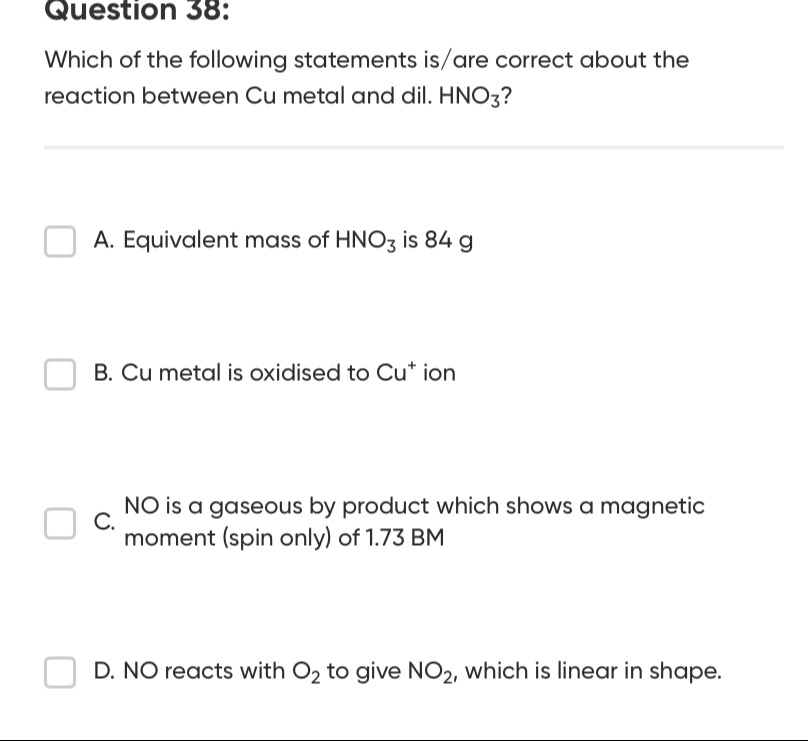
Equivalent mass of HNO3 is 84 g
Cu metal is oxidised to Cu+ ion
NO is a gaseous by product which shows a magnetic moment (spin only) of 1.73 BM
NO reacts with O2 to give NO2, which is linear in shape.
C
Solution
The reaction between copper metal and dilute nitric acid is:
3Cu(s)+8HNO3(dil)→3Cu(NO3)2(aq)+2NO(g)+4H2O(l)
Let's analyze each statement:
A. Equivalent mass of HNO3 is 84 g
In the reaction, nitrogen in HNO3 is in the +5 oxidation state, and in NO, it is in the +2 oxidation state. The change in oxidation state for nitrogen is 5−2=3. The molar mass of HNO3 = 1 (H) + 14 (N) + 3 * 16 (O) = 63 g/mol. The equivalent mass of an oxidizing agent is its molar mass divided by the number of electrons gained per molecule. Equivalent mass of HNO3 = Molar Mass / n-factor = 63 g/mol / 3 = 21 g. Therefore, statement A is incorrect.
B. Cu metal is oxidised to Cu+ ion
In the reaction, copper metal (Cu) has an oxidation state of 0. In the product Cu(NO3)2, copper is in the +2 oxidation state (since NO3− has a -1 charge, and there are two nitrate ions). Thus, Cu metal is oxidized to Cu2+ ion, not Cu+ ion. Therefore, statement B is incorrect.
C. NO is a gaseous byproduct which shows a magnetic moment (spin only) of 1.73 BM
From the balanced chemical equation, NO (nitric oxide) is indeed a gaseous byproduct. To determine its magnetic moment, we look at its electronic configuration. NO has a total of 11 valence electrons (5 from N + 6 from O). According to molecular orbital theory, the electron configuration for NO is: (σ2s)2(σ2s∗)2(σ2pz)2(π2px)2(π2py)2(π2px∗)1 The highest occupied molecular orbital (π2px∗) contains one unpaired electron. The spin-only magnetic moment (μs) is calculated using the formula μs=n(n+2) BM, where n is the number of unpaired electrons. For NO, n = 1. μs=1(1+2)=3≈1.732 BM. Therefore, statement C is correct.
D. NO reacts with O2 to give NO2, which is linear in shape.
Nitric oxide (NO) readily reacts with oxygen (O2) in the air to form nitrogen dioxide (NO2), a reddish-brown gas: 2NO(g)+O2(g)→2NO2(g) This part of the statement is correct. However, let's determine the shape of NO2. NO2 has 17 valence electrons (5 from N + 2*6 from O). It is an odd-electron molecule. The central nitrogen atom in NO2 has two bonding domains (one double bond to an oxygen and one single bond to another oxygen) and one unpaired electron. According to VSEPR theory, these three electron domains result in a bent (V-shaped or angular) geometry, not a linear one. The bond angle in NO2 is approximately 134.3°, which is significantly less than 180° (linear). Therefore, statement D is incorrect.
Conclusion:
Only statement C is correct.
