Question
Question: Which one of the following reactions will not lead to the desired product formation in major proport...
Which one of the following reactions will not lead to the desired product formation in major proportion?
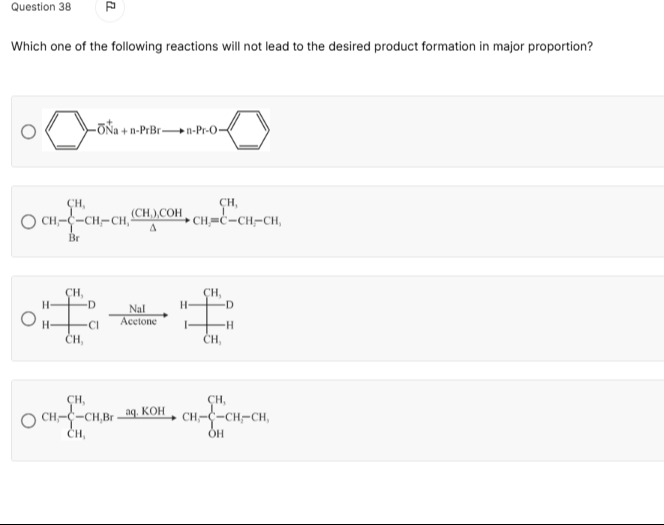
Williamson ether synthesis: Sodium phenoxide reacts with n-propyl bromide.
Elimination reaction: 2-bromo-2-methylbutane reacts with tert-butanol and heat.
Finkelstein reaction: 2-chloro-2-methylpropane reacts with sodium iodide in acetone.
Substitution reaction: 2-bromo-2-methylpropane reacts with aqueous KOH.
Option 3: The reaction in option 3 involves a tertiary alkyl halide reacting with NaI in acetone. This is a Finkelstein reaction, which proceeds via an SN2 mechanism. Tertiary alkyl halides do not undergo SN2 reactions due to steric hindrance. Therefore, the desired product (2-iodo-2-methylpropane) will not be formed in major proportion via SN2.
Solution
The question asks to identify the reaction that will NOT lead to the formation of the depicted product in major proportion. We analyze each reaction:
-
Option 1: This is a Williamson ether synthesis. Sodium phenoxide (a nucleophile) reacts with n-propyl bromide (a primary alkyl halide). This is a typical SN2 reaction, which proceeds efficiently to form the ether, n-propyl phenyl ether, as the major product. Thus, this reaction leads to the desired product in major proportion.
-
Option 2: The substrate is 2-bromo-2-methylbutane, a tertiary alkyl halide. The reagent is tert-butanol and heat, which are conditions for elimination reactions. Elimination of HBr can lead to two alkenes: 2-methylbut-1-ene (less substituted, Hofmann product) and 2-methylbut-2-ene (more substituted, Zaitsev product). According to Zaitsev's rule, the more substituted alkene is generally the major product. The depicted product is 2-methylbut-1-ene. Therefore, the depicted product is likely the minor product, and the reaction will not lead to its formation in major proportion.
-
Option 3: The substrate is 2-chloro-2-methylpropane, a tertiary alkyl halide. The reagent is sodium iodide in acetone, which is a Finkelstein reaction. This reaction proceeds via an SN2 mechanism. However, tertiary alkyl halides do not undergo SN2 reactions due to significant steric hindrance. Therefore, the formation of the depicted product, 2-iodo-2-methylpropane, via SN2 will not occur in major proportion (or at all via SN2). This reaction will not lead to the desired product formation in major proportion.
-
Option 4: The substrate is 2-bromo-2-methylpropane, a tertiary alkyl halide. The reagent is aqueous KOH, a strong base and nucleophile. For tertiary alkyl halides with strong bases, elimination (E2) is generally favored over substitution (SN1). The depicted product is 2-methylpropan-2-ol, which is the substitution product. The major product is expected to be 2-methylpropene, the elimination product. Therefore, the depicted product is likely the minor product, and the reaction will not lead to its formation in major proportion.
Comparing options 2, 3, and 4, option 3 represents a reaction where the depicted mechanism (SN2) is fundamentally incompatible with the substrate (tertiary halide), making the formation of the product via this mechanism impossible. In options 2 and 4, the depicted products are formed, but as minor products due to regioselectivity or competing reaction pathways. However, the question asks for one reaction. In the context of typical exam questions, a reaction that cannot proceed via the implied mechanism is often the intended answer when asked about failure to form a product. Therefore, option 3 is the most definitive answer.
