Question
Question: Match List-I with List-II and choose the correct option...
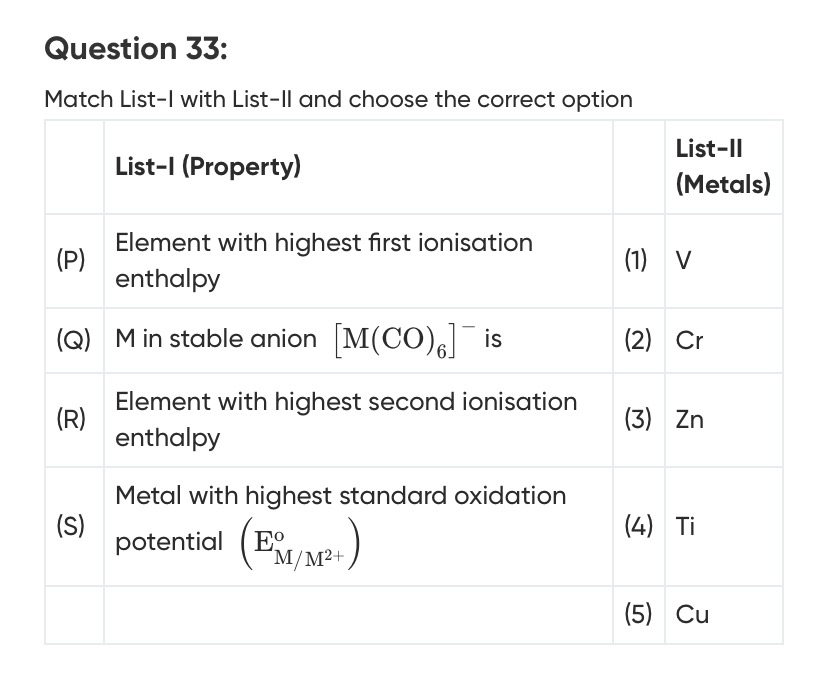
Match List-I with List-II and choose the correct option
P-1, Q-2, R-3, S-4
P-3, Q-1, R-5, S-4
P-2, Q-3, R-1, S-5
P-4, Q-5, R-2, S-3
P-3, Q-1, R-5, S-4
Solution
Explanation:
(P) Element with highest first ionization enthalpy: The first ionization enthalpy generally increases across a period due to increasing nuclear charge. However, exceptions occur due to stable electron configurations.
- Ti: [Ar] 3d² 4s²
- V: [Ar] 3d³ 4s²
- Cr: [Ar] 3d⁵ 4s¹ (stable half-filled d-subshell)
- Cu: [Ar] 3d¹⁰ 4s¹ (stable fully-filled d-subshell)
- Zn: [Ar] 3d¹⁰ 4s² (stable fully-filled d and s subshells)
The first ionization enthalpy involves removing the outermost electron. Zn has the electronic configuration [Ar] 3d¹⁰ 4s². Removing one 4s electron leaves it with a stable [Ar] 3d¹⁰ 4s¹ configuration. The combined stability of the filled 3d and 4s subshells leads to the highest first ionization enthalpy for Zn among the given metals. Approximate IE1 values (kJ/mol): Ti (656), V (650), Cr (653), Zn (906), Cu (745). Therefore, (P) matches with (3) Zn.
(Q) M in stable anion [M(CO)6]−: For stability, metal carbonyl complexes often follow the 18-electron rule. The electron count is calculated as: Electron count = (Valence electrons of M) + 2 × (Number of CO ligands) - (Charge of the complex) Here, the complex is [M(CO)6]−, with 6 CO ligands (neutral) and a net charge of -1. Electron count = (Valence electrons of M) + 2 × 6 - (-1) = Valence electrons of M + 12 + 1 = Valence electrons of M + 13. For the 18-electron rule, Valence electrons of M + 13 = 18, which means Valence electrons of M = 5. Vanadium (V) is in Group 5 and has 5 valence electrons ([Ar] 3d³ 4s²). So, for V, the electron count is 5 + 13 = 18. [V(CO)6]− is a known stable complex. Therefore, (Q) matches with (1) V.
(R) Element with highest second ionization enthalpy: The second ionization enthalpy (IE2) is the energy required to remove the second electron from a neutral atom (i.e., to form M²⁺ from M⁺). High IE2 is observed when the M⁺ ion has a particularly stable electronic configuration.
- Cr: [Ar] 3d⁵ 4s¹ → IE1 → Cr⁺ [Ar] 3d⁵ (stable) → IE2 → Cr²⁺ [Ar] 3d⁴
- Cu: [Ar] 3d¹⁰ 4s¹ → IE1 → Cu⁺ [Ar] 3d¹⁰ (very stable) → IE2 → Cu²⁺ [Ar] 3d⁹
- Zn: [Ar] 3d¹⁰ 4s² → IE1 → Zn⁺ [Ar] 3d¹⁰ 4s¹ → IE2 → Zn²⁺ [Ar] 3d¹⁰ (very stable)
While Zn²⁺ is very stable, the IE2 for Cu is generally higher because Cu⁺ ([Ar] 3d¹⁰) is exceptionally stable, and the removal of the second electron (from the 3d subshell) is energetically demanding. Approximate IE2 values (kJ/mol): Ti (1300), V (1400), Cr (1890), Cu (1958), Zn (1733). Cu has the highest second ionization enthalpy among these. Therefore, (R) matches with (5) Cu.
(S) Metal with highest standard oxidation potential (EM/M2+o): The standard oxidation potential (EM/M2+o) is the negative of the standard reduction potential (EM2+/Mo). A higher oxidation potential means the metal is more easily oxidized. This corresponds to a more negative reduction potential. Standard Reduction Potentials (EM2+/Mo):
- Ti: -1.63 V
- V: -1.18 V
- Cr: -0.91 V
- Cu: +0.34 V
- Zn: -0.76 V
The most negative reduction potential is -1.63 V for Titanium. Therefore, the standard oxidation potential for Titanium is ETi/Ti2+o=−(−1.63 V)=+1.63 V, which is the highest among the given metals. Therefore, (S) matches with (4) Ti.
Summary of Matches: (P) → (3) Zn (Q) → (1) V (R) → (5) Cu (S) → (4) Ti
The correct option is the one that represents these matches: P-3, Q-1, R-5, S-4.
