Question
Question: For a first order reaction $A \longrightarrow \frac{1}{n}B$, the variation of concentration of (A) a...
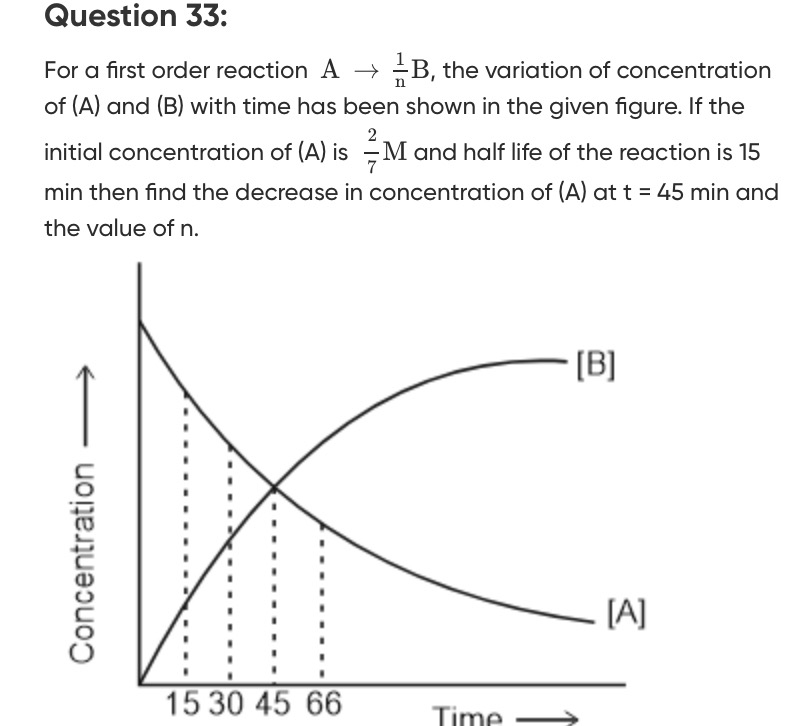
For a first order reaction A⟶n1B, the variation of concentration of (A) and (B) with time has been shown in the given figure. If the initial concentration of (A) is 72M and half life of the reaction is 15 min then find the decrease in concentration of (A) at t = 45 min and the value of n.
Decrease in concentration of (A) at t = 45 min is 41M and the value of n is 7.
Solution
The problem involves a first-order reaction and requires calculating the decrease in concentration of a reactant and determining a stoichiometric coefficient using half-life and graphical information.
1. Calculate the decrease in concentration of (A) at t = 45 min: For a first-order reaction, the half-life (t1/2) is constant. Given t1/2=15 min. The time elapsed is t=45 min. The number of half-lives that have passed is m=t1/2t=15 min45 min=3.
For a first-order reaction, the concentration of reactant remaining after 'm' half-lives is given by: [A]t=2m[A]0 Given initial concentration [A]0=72M. At t=45 min (after 3 half-lives): [A]45=23[A]0=8[A]0 [A]45=81×72M=41×71M=281M.
The decrease in concentration of (A) is the initial concentration minus the concentration at time t: Decrease in [A]=[A]0−[A]45 Decrease in [A]=72M−281M To subtract, find a common denominator (28): Decrease in [A]=7×42×4M−281M=288M−281M=287M=41M.
2. Find the value of n: The reaction is A⟶n1B. From the graph, at t=45 min, the concentration of A and B are equal (the curves intersect). So, [B]45=[A]45=281M.
Let x be the amount of A reacted at t=45 min. x=[A]0−[A]45=41M.
According to the stoichiometry of the reaction A⟶n1B: If 1 mole of A reacts, n1 moles of B are formed. If x moles of A react, then n1x moles of B are formed. So, [B]45=n1x.
Substitute the known values: 281M=n1×41M 281=4n1 Cross-multiply: 4n=28 n=428 n=7.
