Question
Question: On treatment of 100ml of 0.1 M solution of complex CrCl3.6H₂O with excess of AgNO3,4.305 g of AgCl w...
On treatment of 100ml of 0.1 M solution of complex CrCl3.6H₂O with excess of AgNO3,4.305 g of AgCl was obtained the complex is
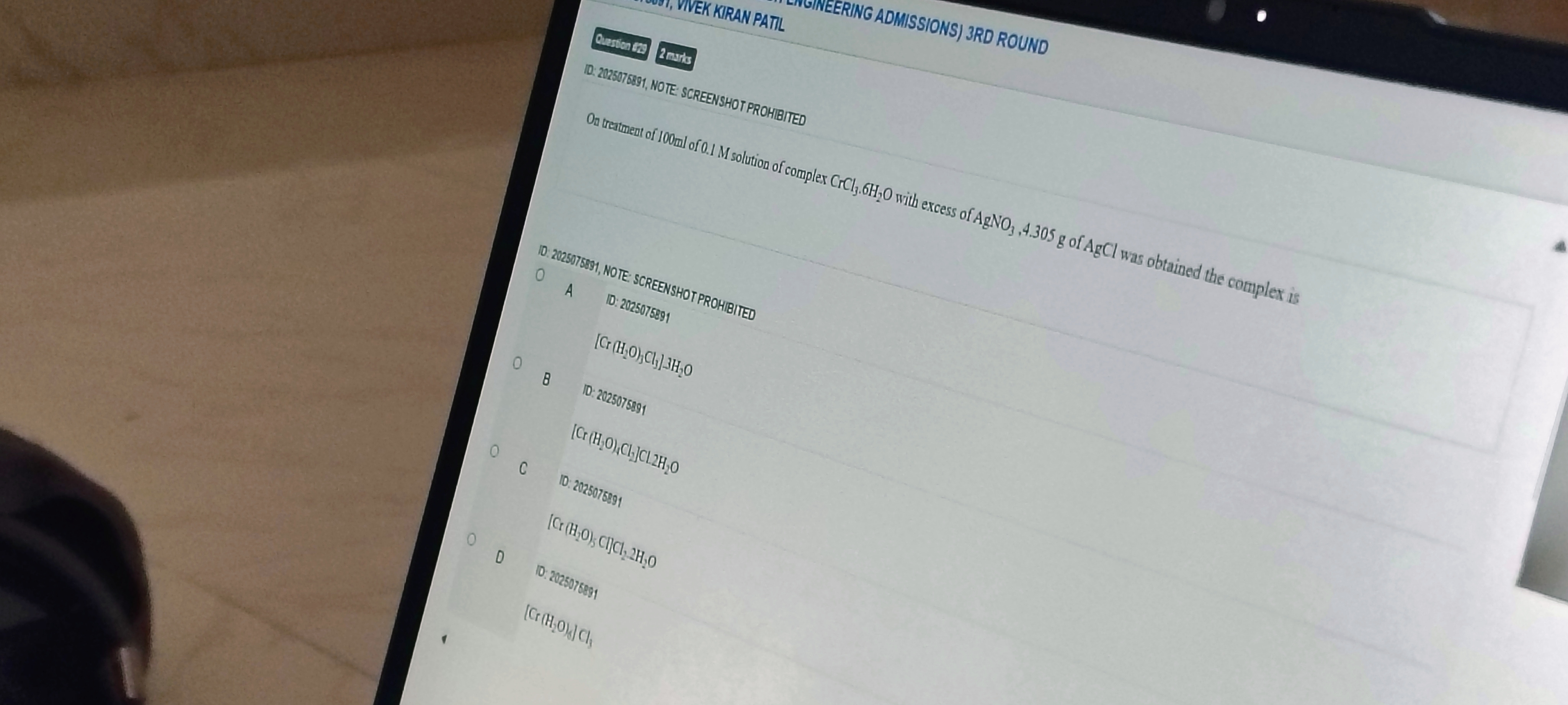
[Cr (H₂O)₆Cl₃].3H₂O
[Cr (H₂O)₄Cl₄]Cl.2H₂O
[Cr (H₂O)₅ Cl]Cl₂.2H₂O
[Cr (H₂O)₆] Cl₃
D
Solution
-
1. Calculate moles of the complex:
Given volume = 100 mL = 0.1 L
Given concentration = 0.1 M
Moles of complex = Volume × Concentration = 0.1 L × 0.1 mol/L = 0.01 mol -
2. Calculate moles of AgCl obtained:
Given mass of AgCl = 4.305 g
Molar mass of AgCl (Ag = 107.87 g/mol, Cl = 35.45 g/mol) = 107.87 + 35.45 = 143.32 g/mol
Moles of AgCl = Mass / Molar mass = 4.305 g / 143.32 g/mol ≈ 0.03 mol -
3. Determine the number of ionizable chloride ions:
The reaction between the complex and AgNO₃ involves the precipitation of AgCl from the ionizable chloride ions (those outside the coordination sphere).
The mole ratio of AgCl to the complex indicates the number of ionizable Cl⁻ ions per molecule of the complex.
Number of ionizable Cl⁻ ions = Moles of AgCl / Moles of complex = 0.03 mol / 0.01 mol = 3 -
4. Identify the correct complex formula from the options:
The general formula of the complex is CrCl₃.6H₂O, meaning it contains 1 Cr, 3 Cl, and 6 H₂O in total. We are looking for an option that has 3 ionizable Cl⁻ ions and matches the overall composition.
Let's check each option:
- A: [Cr (H₂O)₆Cl₃].3H₂O
Total Cl = 3 (all inside, so 0 ionizable). Total H₂O = 6 (inside) + 3 (outside) = 9. (Incorrect total H₂O and ionizable Cl) - B: [Cr (H₂O)₄Cl₄]Cl.2H₂O
Total Cl = 4 (inside) + 1 (outside) = 5. (Incorrect total Cl) - C: [Cr (H₂O)₅ Cl]Cl₂.2H₂O
Total Cl = 1 (inside) + 2 (outside) = 3. Ionizable Cl = 2. Total H₂O = 5 (inside) + 2 (outside) = 7. (Incorrect total H₂O and ionizable Cl) - D: [Cr (H₂O)₆] Cl₃
Total Cl = 0 (inside) + 3 (outside) = 3. Ionizable Cl = 3. Total H₂O = 6 (inside) + 0 (outside) = 6. (Matches all criteria)
Only option D correctly accounts for 3 ionizable chloride ions and the overall composition of CrCl₃.6H₂O.
- A: [Cr (H₂O)₆Cl₃].3H₂O
The final answer is D
Explanation of the solution:
The moles of the complex CrCl₃.6H₂O are calculated as 0.1 L × 0.1 M = 0.01 mol. The moles of AgCl precipitated are calculated as 4.305 g / 143.32 g/mol ≈ 0.03 mol. The ratio of moles of AgCl to moles of complex is 0.03 / 0.01 = 3. This indicates that there are 3 ionizable chloride ions per molecule of the complex. Among the given options, only [Cr(H₂O)₆]Cl₃ has 3 chloride ions outside the coordination sphere (ionizable) and also correctly represents the total number of Cr, Cl, and H₂O atoms/molecules as per CrCl₃.6H₂O.
