Question
Question: Binding energy of electrons in a metal is 250 KJ $mol^{-1}$. Then threshold frequency of metal is...
Binding energy of electrons in a metal is 250 KJ mol−1. Then threshold frequency of metal is
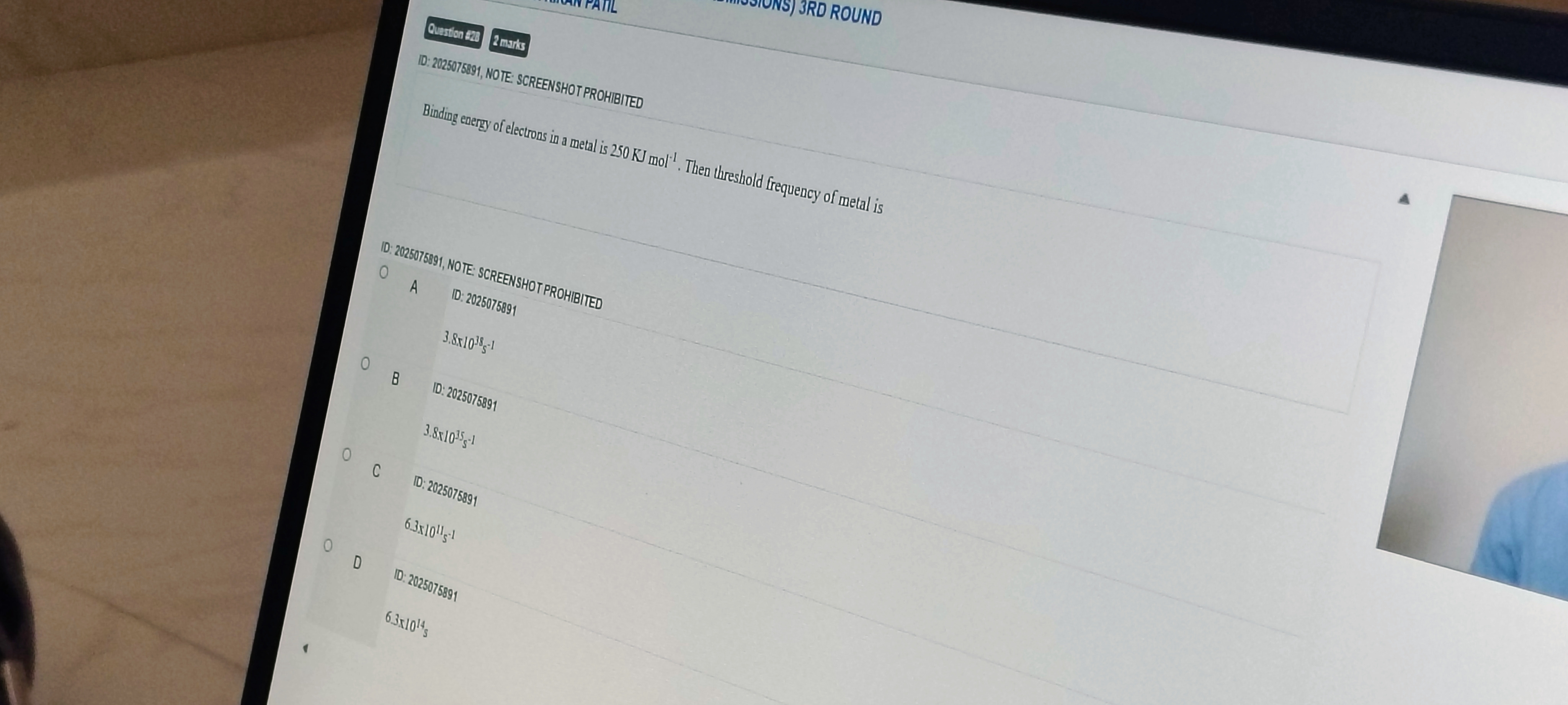
ID: 2025075891 3.8x1038s−1
ID: 2025075891 3.8x1035s−1
ID: 2025075891 6.3x1011s−1
ID: 2025075891 6.3x1014s
D
Solution
The binding energy of electrons in a metal is given as 250 KJ mol−1. This binding energy is equivalent to the work function (Φ) per mole. To find the work function for a single electron, we need to divide the molar binding energy by Avogadro's number (NA).
-
Convert molar binding energy to Joules per mole: Binding energy = 250 KJ mol−1=250×103 J mol−1
-
Calculate the work function (Φ) per electron: The work function (Φ) is the minimum energy required to eject one electron. Φ=NABinding energy per mole Using Avogadro's number (NA=6.022×1023 mol−1): Φ=6.022×1023 mol−1250×103 J mol−1 Φ=6.022×10232.5×105 J Φ≈4.151×10−19 J
-
Calculate the threshold frequency (ν0): The work function is related to the threshold frequency by Planck's equation: Φ=hν0 Where h is Planck's constant (h=6.626×10−34 J s). Rearranging the formula to find ν0: ν0=hΦ ν0=6.626×10−34 J s4.151×10−19 J ν0≈0.6265×1015 s−1 ν0≈6.265×1014 s−1
-
Compare with given options: Rounding the calculated value to one decimal place, we get 6.3×1014 s−1. Option D is 6.3×1014 s. Although the unit is incorrectly given as 's' (which is for period) instead of 's−1' (which is for frequency), the numerical value matches our calculation. It is a common occurrence in multiple-choice questions for there to be a typo in the unit, but the numerical value is intended to be correct.
