Question
Question: How many formula of complex can form more than two optically active isomers (where M is metal ion, a...
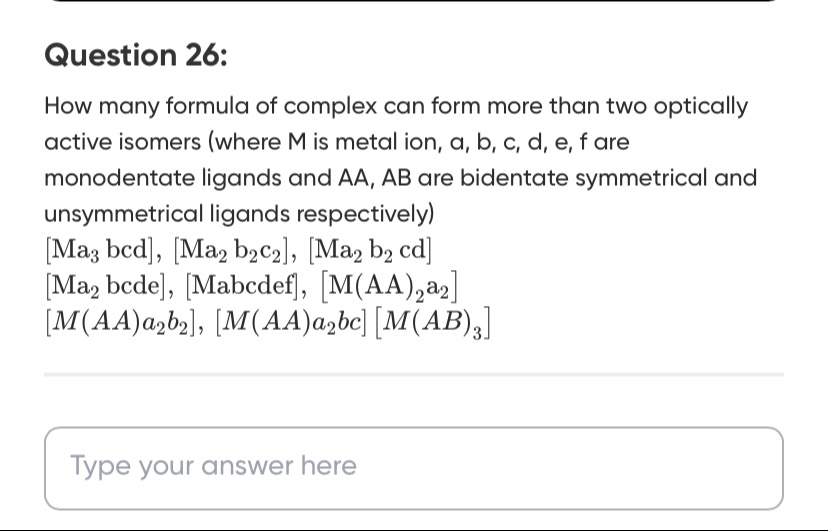
How many formula of complex can form more than two optically active isomers (where M is metal ion, a, b, c, d, e, f are monodentate ligands and AA, AB are bidentate symmetrical and unsymmetrical ligands respectively)
A
[Ma3bcd]
B
[Ma2b2c2]
C
[Ma2b2cd]
D
[Ma2bcde]
E
[Mabcdef]
F
[M(AA)2a2]
G
[M(AA)a2b2]
H
[M(AA)a2bc]
I
[M(AB)3]
Answer
5
Explanation
Solution
The question asks to identify coordination complexes that can form more than two optically active isomers. A complex is optically active if it is chiral. We evaluate each complex formula for optical activity:
- [Ma3bcd]: Forms 6 optically active isomers. (Qualifies)
- [Ma2b2c2]: Forms 0 optically active isomers. (Does not qualify)
- [Ma2b2cd]: Forms 4 optically active isomers. (Qualifies)
- [Ma2bcde]: Forms 6 optically active isomers. (Qualifies)
- [Mabcdef]: Forms 30 optically active isomers. (Qualifies)
- [M(AA)2a2]: Forms 2 optically active isomers (from cis isomer). (Does not qualify as it's not more than two)
- [M(AA)a2b2]: Forms 2 optically active isomers. (Does not qualify)
- [M(AA)a2bc]: Forms 4 optically active isomers. (Qualifies)
- [M(AB)3]: Forms 2 optically active isomers (from cis isomer). (Does not qualify)
The complexes that form more than two optically active isomers are [Ma3bcd], [Ma2b2cd], [Ma2bcde], [Mabcdef], and [M(AA)a2bc]. There are 5 such complexes.
