Question
Question: Which one of the following statements is not consistent with the kinetic molecular theory of gases?...
Which one of the following statements is not consistent with the kinetic molecular theory of gases?
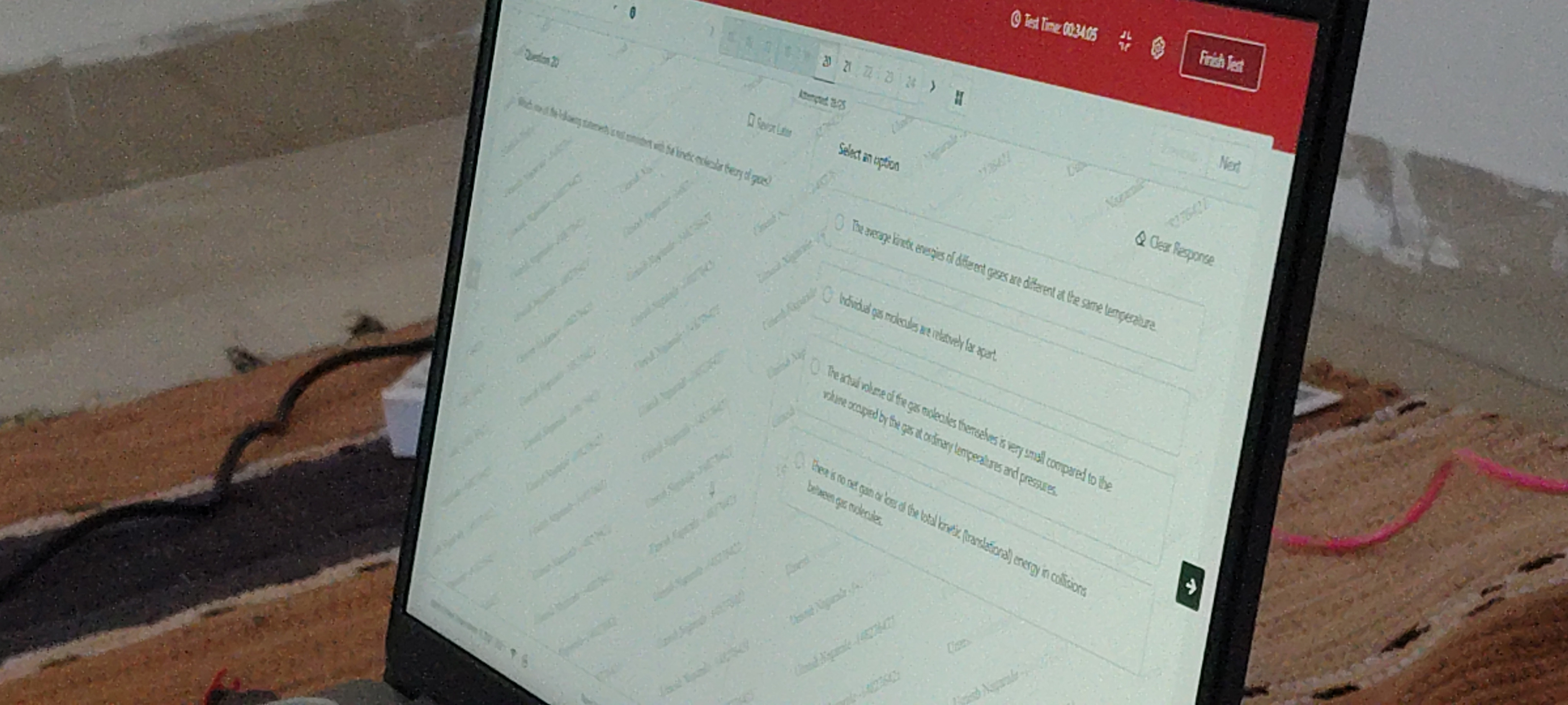
The average kinetic energies of different gases are different at the same temperature.
Individual gas molecules are relatively far apart.
The actual volume of the gas molecules themselves is very small compared to the volume occupied by the gas at ordinary temperatures and pressures.
There is no net gain or loss of the total kinetic (translational) energy in collisions between gas molecules.
The average kinetic energies of different gases are different at the same temperature.
Solution
The Kinetic Molecular Theory (KMT) posits that the average kinetic energy of gas molecules is directly proportional to the absolute temperature, described by the formula KEavg=23kT, where 'k' is the Boltzmann constant and 'T' is the absolute temperature. This implies that at a given temperature, all gases have the same average kinetic energy, regardless of their chemical identity or molar mass. Therefore, the statement that average kinetic energies of different gases are different at the same temperature is inconsistent with KMT.
