Question
Question: Calculate magnitude of work done in calorie by one mole of an ideal gas subjected to the process as ...
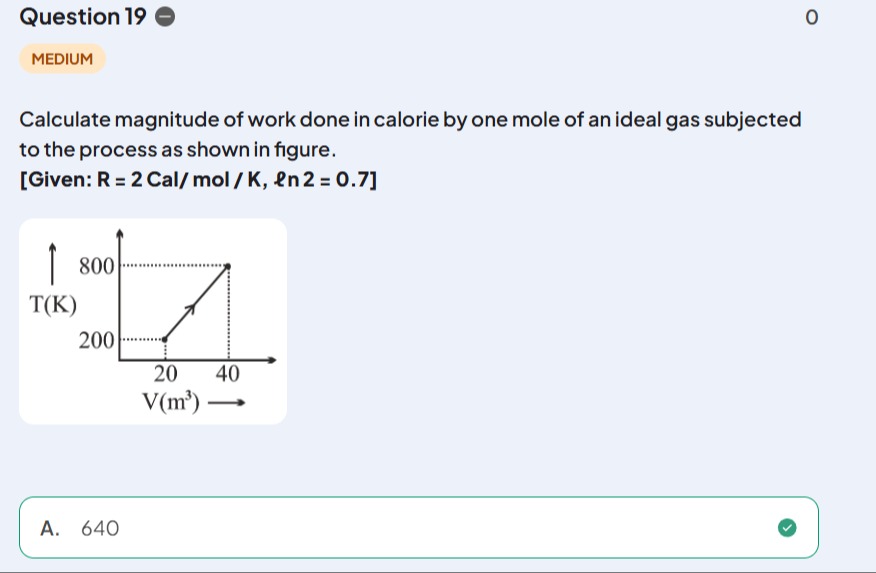
Calculate magnitude of work done in calorie by one mole of an ideal gas subjected to the process as shown in figure. [Given: R = 2 Cal/mol / K, ln2=0.7]
640
640
Solution
The work done by an ideal gas is given by W=∫PdV. From the ideal gas law, PV=nRT, so P=VnRT. The process is represented by a straight line in the T-V diagram, passing through points (V1,T1)=(20 m3,200 K) and (V2,T2)=(40 m3,800 K). The equation of the line is T=mV+c. The slope is m=V2−V1T2−T1=40−20800−200=20600=30 K/m3. Using point (20,200): 200=30(20)+c⟹200=600+c⟹c=−400 K. So, T=30V−400.
Now, substitute P in the work integral: W=∫V1V2VnRTdV W=nR∫V1V2V30V−400dV W=nR∫V1V2(30−V400)dV W=nR[30V−400ln∣V∣]V1V2 W=nR[(30V2−400lnV2)−(30V1−400lnV1)] W=nR[30(V2−V1)−400(lnV2−lnV1)] W=nR[30(V2−V1)−400ln(V1V2)]
Given n=1 mol, R=2 Cal/mol/K, V1=20 m³, V2=40 m³, and ln2=0.7. W=(1 mol)×(2 Cal/mol/K)[30(40 m3−20 m3)−400ln(20 m340 m3)] W=2 Cal/K[30(20 m3)−400ln(2)] W=2 Cal/K[600 K−400×0.7] W=2 Cal/K[600 K−280 K] W=2 Cal/K[320 K] W=640 Cal.
Since the volume increases, the work done by the gas is positive. The magnitude of work done is therefore 640 Cal.
