Question
Question: Which of the following is not correct?...
Which of the following is not correct?
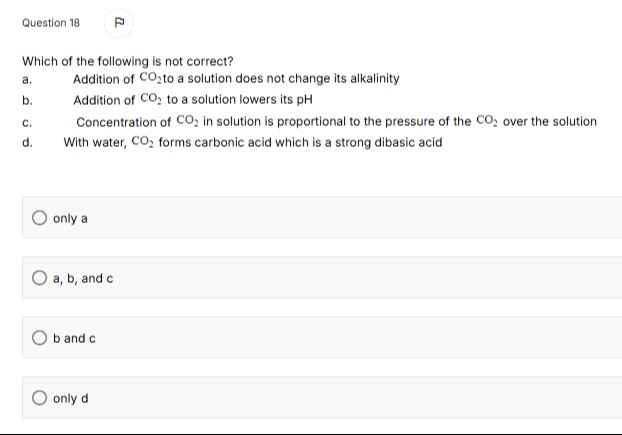
Addition of CO₂ to a solution does not change its alkalinity
Addition of CO₂ to a solution lowers its pH
Concentration of CO₂ in solution is proportional to the pressure of the CO₂ over the solution
With water, CO₂ forms carbonic acid which is a strong dibasic acid
only d
Solution
-
(a) When CO₂ is added to a solution, it forms carbonic acid, which dissociates into H⁺ and HCO₃⁻. The H⁺ produced is balanced by the formation of bicarbonate, so the overall alkalinity (defined as the capacity to neutralize acid) remains unchanged. Thus, statement (a) is correct.
-
(b) Addition of CO₂ increases the concentration of carbonic acid, thereby increasing the H⁺ concentration and lowering the pH. So, (b) is correct.
-
(c) According to Henry’s law, the concentration of a gas dissolved in a liquid is proportional to its partial pressure. Hence, (c) is correct.
-
(d) Although CO₂ with water forms carbonic acid (H₂CO₃), it is a weak acid, not a strong dibasic acid. Thus, statement (d) is not correct.
The only incorrect statement is (d).
