Question
Question: 1 moles of the He gas undergoes a cyclic process *MNOPM* as shown in *T - V* diagram. Temperature of...
1 moles of the He gas undergoes a cyclic process MNOPM as shown in T - V diagram. Temperature of gas in state M is 300 K.
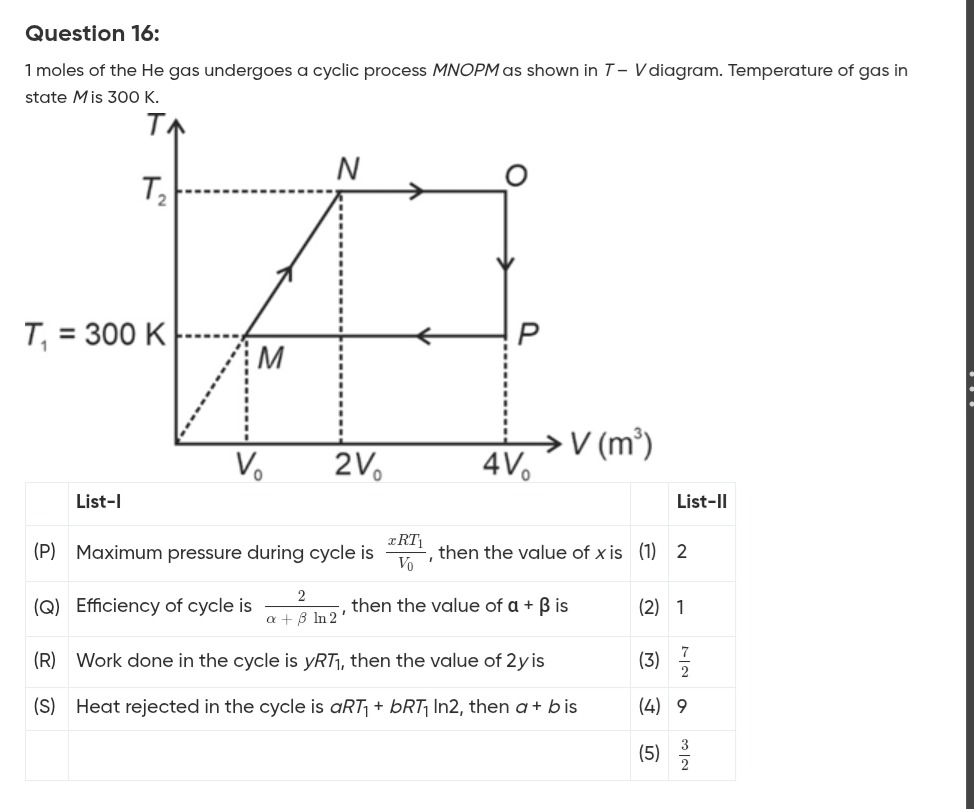
Maximum pressure during cycle is V0xRT1, then the value of x is
Efficiency of cycle is α+βln22, then the value of α+β is
Work done in the cycle is yRT1, then the value of 2y is
Heat rejected in the cycle is aRT1+bRT1ln2, then a + b is
(P) - (2), (Q) - (4), (R) - (1), (S) - (3)
Solution
The given process is a cyclic process MNOPM for 1 mole of He gas (monoatomic, CV=23R, CP=25R). The temperature at state M is T1=300 K.
From the T-V diagram:
State M: (V0,T1)
State N: (2V0,T2)
State O: (4V0,T2)
State P: (4V0,T1)
Process M-N is a straight line in T-V diagram passing through the origin if extended, so T∝V. Thus, VMTM=VNTN, so V0T1=2V0T2, which gives T2=2T1.
Also, P=VRT. Since VT is constant, P is constant. Process M-N is isobaric.
PMN=VMRTM=V0RT1.
Process N-O is isothermal at T2=2T1.
Process O-P is isochoric at VO=4V0.
Process P-M is isothermal at T1.
Pressures at the states:
PM=V0RT1
PN=2V0RT2=2V0R(2T1)=V0RT1
PO=4V0RT2=4V0R(2T1)=2V0RT1
PP=4V0RT1
(P) Maximum pressure during cycle.
Pressures at the vertices are PM=PN=V0RT1, PO=2V0RT1, PP=4V0RT1.
During N-O (isothermal expansion), P=V2RT1, pressure decreases from PN to PO.
During O-P (isochoric cooling), P=4V0RT, pressure decreases from PO to PP.
During P-M (isothermal compression), P=VRT1, pressure increases from PP to PM.
Maximum pressure is V0RT1. Given maximum pressure is V0xRT1, so x=1.
(P) matches with (2).
(Q) Efficiency of cycle η=QinWcycle.
Work done in each process:
WMN=PMN(VN−VM)=V0RT1(2V0−V0)=RT1.
WNO=nRTNln(VNVO)=R(2T1)ln(2V04V0)=2RT1ln2.
WOP=0 (isochoric).
WPM=nRTPln(VPVM)=RT1ln(4V0V0)=RT1ln(4−1)=−RT1ln4=−2RT1ln2.
Wcycle=WMN+WNO+WOP+WPM=RT1+2RT1ln2+0−2RT1ln2=RT1.
Heat transfer in each process (n=1):
QMN=nCP(TN−TM)=1×25R(2T1−T1)=25RT1 (heat absorbed).
QNO=WNO=2RT1ln2 (heat absorbed, isothermal expansion).
QOP=nCV(TP−TO)=1×23R(T1−2T1)=−23RT1 (heat rejected).
QPM=WPM=−2RT1ln2 (heat rejected, isothermal compression).
Heat absorbed Qin=QMN+QNO=25RT1+2RT1ln2.
Efficiency η=QinWcycle=25RT1+2RT1ln2RT1=25+2ln21=5+4ln22.
Given efficiency is α+βln22. Comparing, α=5,β=4.
α+β=5+4=9.
(Q) matches with (4).
(R) Work done in the cycle is yRT1.
We found Wcycle=RT1. Given Wcycle=yRT1, so y=1.
We need to find 2y=2×1=2.
(R) matches with (1).
(S) Heat rejected in the cycle is aRT1+bRT1ln2.
Heat rejected Qrejected=∣QOP∣+∣QPM∣=∣−23RT1∣+∣−2RT1ln2∣=23RT1+2RT1ln2.
Given heat rejected is aRT1+bRT1ln2. Comparing, a=23 and b=2.
a+b=23+2=23+24=27.
(S) matches with (3).
Summary of matches:
(P) - (2)
(Q) - (4)
(R) - (1)
(S) - (3)
Explanation of the solution:
-
Determine the coordinates (V, T) for each state M, N, O, P from the T-V diagram.
-
Identify the type of process for each step (M-N, N-O, O-P, P-M) based on the T-V diagram.
-
Calculate the pressure at each state using the ideal gas law PV=nRT.
-
For (P), find the maximum pressure during the cycle by examining the pressures at the states and how pressure changes during each process. Compare with the given expression to find x.
-
For (Q), calculate the work done and heat absorbed/rejected in each process. Calculate the net work done in the cycle and the total heat absorbed. Calculate the efficiency η=QinWcycle. Compare with the given expression to find α and β, then calculate α+β.
-
For (R), the work done in the cycle is already calculated in step 5. Compare with the given expression to find y, then calculate 2y.
-
For (S), identify the processes where heat is rejected. Calculate the magnitude of heat rejected in these processes and sum them up. Compare with the given expression to find a and b, then calculate a+b.
-
Match the calculated values with the options in List-II.
