Question
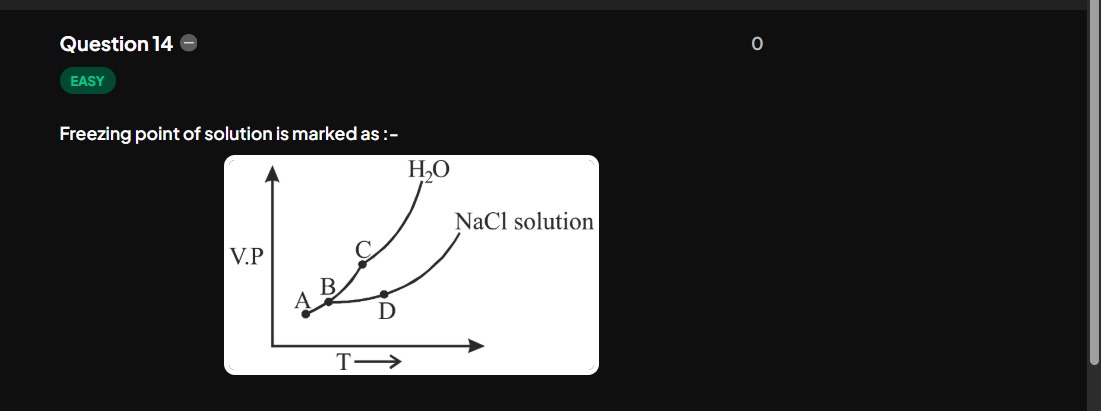
Question: Freezing point of solution is marked as :- $H_2O$ NaCl solution...
Freezing point of solution is marked as :-
H2O
NaCl solution
Answer
A
Explanation
Solution
The freezing point of a solution is lower than that of the pure solvent (freezing point depression). In the given vapor pressure-temperature graph, the upper curve represents pure H2O and the lower curve represents the NaCl solution. Point B is the freezing point of pure H2O. Due to freezing point depression, the freezing point of the NaCl solution must be at a lower temperature than B. Point A is on the NaCl solution curve and is at a lower temperature than B. Thus, A represents the freezing point of the NaCl solution.
