Question
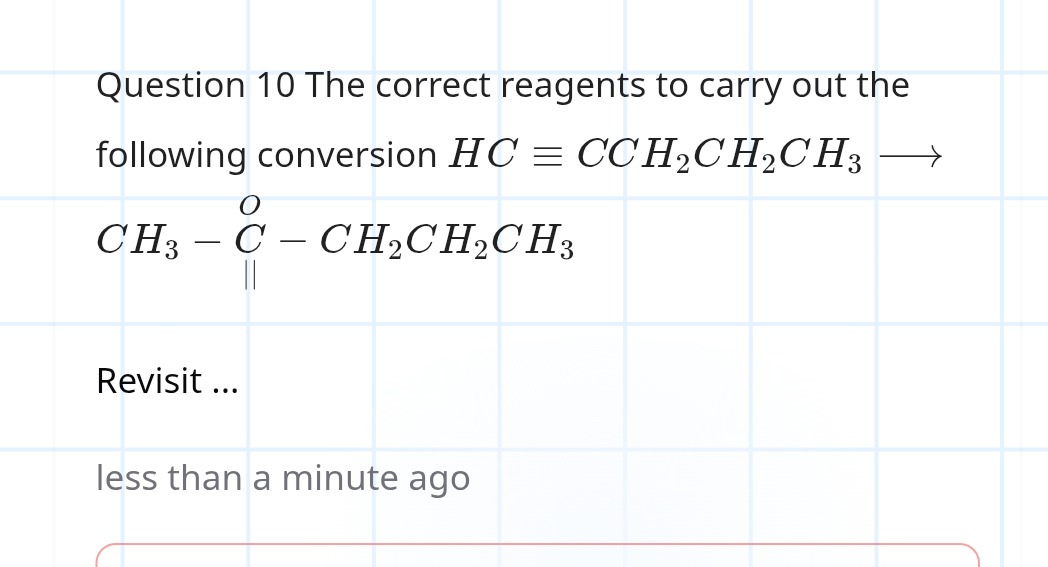
Question: The correct reagents to carry out the following conversion $HC \equiv CCH_2CH_2CH_3 \rightarrow CH_3...
The correct reagents to carry out the following conversion HC≡CCH2CH2CH3→CH3−∣∣CO−CH2CH2CH3
HgSO_4, H_2SO_4
Solution
The conversion is from 1-pentyne (HC≡CCH2CH2CH3) to 2-pentanone (CH3−∣∣CO−CH2CH2CH3). This is a hydration reaction of a terminal alkyne.
There are two main methods for the hydration of alkynes:
-
Acid-catalyzed hydration with Mercury(II) sulfate (HgSO4) and Sulfuric acid (H2SO4):
This reaction follows Markovnikov's rule, meaning the hydroxyl group (OH) adds to the more substituted carbon of the alkyne and the hydrogen adds to the less substituted carbon. For terminal alkynes, this typically leads to the formation of a ketone (except for acetylene, which gives acetaldehyde).
For 1-pentyne:
HC≡CCH2CH2CH3HgSO4,H2SO4,H2O
The OH adds to C-2 and H adds to C-1, forming an enol:
CH2=C(OH)CH2CH2CH3 (enol form)
This enol rapidly tautomerizes to the more stable keto form:
CH2=C(OH)CH2CH2CH3⇌CH3−∣∣CO−CH2CH2CH3 (2-pentanone)
This matches the desired product.
-
Hydroboration-oxidation (BH3⋅THF followed by H2O2/OH−):
This reaction follows an anti-Markovnikov addition, meaning the hydroxyl group (OH) adds to the less substituted carbon of the alkyne. For terminal alkynes, this leads to the formation of an aldehyde.
For 1-pentyne:
HC≡CCH2CH2CH31.BH3⋅THF2.H2O2/OH−
This would lead to an enol with the OH on C-1:
HO−CH=CHCH2CH2CH3 (enol form)
This enol would tautomerize to an aldehyde:
O=CH−CH2CH2CH2CH3 (pentanal)
This is not the desired product (2-pentanone).
Therefore, the correct reagents to carry out the conversion of 1-pentyne to 2-pentanone are HgSO4 and H2SO4 (in the presence of water).
The reaction can be represented as:
HC≡CCH2CH2CH3HgSO4,H2SO4,H2OCH3−∣∣CO−CH2CH2CH3Explanation:
The conversion of 1-pentyne to 2-pentanone requires the hydration of a terminal alkyne following Markovnikov's rule. Acid-catalyzed hydration using HgSO4/H2SO4 achieves this, forming an enol intermediate that tautomerizes to the desired ketone. Hydroboration-oxidation would yield an aldehyde (pentanal) via anti-Markovnikov addition.
