Question
Question: $\qquad ^{+}O-H$...
+O−H
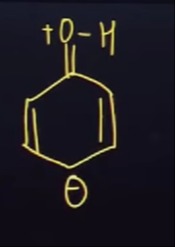
The cyclic system is aromatic due to the presence of 6 π electrons in a planar, conjugated ring.
Solution
The given structure depicts a six-membered ring containing two double bonds and a negative charge, with a protonated carbonyl group (=O+H) attached to one of the ring carbons. The two double bonds contribute 4 π electrons to the ring system. The negative charge, if delocalized into the ring, contributes an additional 2 π electrons. Thus, the cyclic system has a total of 4+2=6 π electrons. According to Hückel's rule, a planar, cyclic, conjugated system with 4n+2 π electrons is aromatic. In this case, 4n+2=6, which means n=1. Therefore, if the ring is planar, the species is aromatic, exhibiting enhanced stability. The protonated carbonyl group is a resonance contributor, with the positive charge residing on the oxygen atom. The negative charge is delocalized within the ring, contributing to the aromatic character.
