Question
Question: An organic compound [X] (Molecular formula C6H12O2) reacts with dil. H2SO4 to form an alcohol [B] an...
An organic compound [X] (Molecular formula C6H12O2) reacts with dil. H2SO4 to form an alcohol [B] and a carboxylic acid [C]. Reaction of compound [B] with Jones reagent yielded compound [C]. When compound [C] was heated with P2O5 an Anhydride was formed. Compound [X] is ---------.
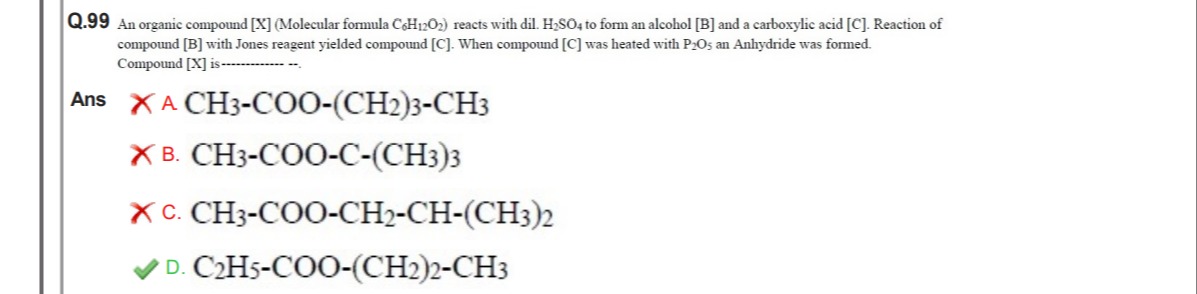
CH3-COO-(CH2)3-CH3
CH3-COO-C-(CH3)3
CH3-COO-CH2-CH-(CH3)2
C2H5-COO-(CH2)2-CH3
D
Solution
The problem describes a series of reactions starting from an organic compound [X] with molecular formula C6H12O2.
-
Hydrolysis of [X]: Compound [X] (C6H12O2) reacts with dilute H2SO4 to form an alcohol [B] and a carboxylic acid [C]. This indicates that [X] is an ester, as esters hydrolyze under acidic conditions to yield a carboxylic acid and an alcohol. Let [X] be R-COO-R'. R-COO-R' + H2O dil. H2SO4 R-COOH ([C], carboxylic acid) + R'-OH ([B], alcohol)
-
Oxidation of [B]: Alcohol [B] reacts with Jones reagent (a strong oxidizing agent) to yield carboxylic acid [C]. R'-OH Jones reagent R-COOH For an alcohol to be oxidized to a carboxylic acid, it must be a primary alcohol. If [B] is R'-OH, and its oxidation product is R-COOH, then the carbon skeleton of [B] must correspond to [C] such that the -CH2OH group in [B] becomes the -COOH group in [C]. This means R' must be R-CH2-. So, [B] is R-CH2-OH. And [C] is R-COOH. Therefore, the ester [X] is R-COO-CH2-R.
-
Dehydration of [C]: Carboxylic acid [C] when heated with P2O5 forms an anhydride. P2O5 is a dehydrating agent, and carboxylic acids form anhydrides upon dehydration. 2 R-COOH P2O5, Δ (R-CO)2O + H2O This confirms [C] is a carboxylic acid.
Determining the structure of R: The molecular formula of [X] is C6H12O2. We deduced that [X] has the structure R-COO-CH2-R. Let 'n' be the number of carbon atoms in the alkyl group R. The total number of carbon atoms in R-COO-CH2-R is: n (from first R) + 1 (from -COO-) + 1 (from -CH2-) + n (from second R) = 2n + 2. Given that [X] has 6 carbon atoms: 2n + 2 = 6 2n = 4 n = 2 So, R is an alkyl group with 2 carbon atoms, i.e., R = C2H5- (ethyl group).
Identifying the compounds:
- [C] (Carboxylic acid): R-COOH = C2H5-COOH = CH3CH2COOH (Propanoic acid)
- [B] (Alcohol): R-CH2-OH = C2H5-CH2-OH = CH3CH2CH2OH (Propan-1-ol)
- [X] (Ester): R-COO-CH2-R = C2H5-COO-CH2-C2H5 = CH3CH2COOCH2CH2CH3 (Propyl propanoate)
Verification:
- Molecular formula of Propyl propanoate (CH3CH2COOCH2CH2CH3): C6H12O2. (Matches)
- Hydrolysis: CH3CH2COOCH2CH2CH3 + H2O → CH3CH2COOH (Propanoic acid) + CH3CH2CH2OH (Propan-1-ol). (Matches [C] and [B])
- Oxidation: CH3CH2CH2OH (Propan-1-ol) Jones reagent CH3CH2COOH (Propanoic acid). (Matches [C])
- Dehydration: 2 CH3CH2COOH P2O5, Δ (CH3CH2CO)2O (Propanoic anhydride). (Matches)
Comparing with options: A. CH3-COO-(CH2)3-CH3 (Butyl acetate): Hydrolysis yields acetic acid and butan-1-ol. Oxidation of butan-1-ol yields butanoic acid, not acetic acid. (Incorrect) B. CH3-COO-C-(CH3)3 (tert-Butyl acetate): Hydrolysis yields acetic acid and tert-butyl alcohol. tert-Butyl alcohol is a tertiary alcohol and does not oxidize to a carboxylic acid under these conditions. (Incorrect) C. CH3-COO-CH2-CH-(CH3)2 (Isobutyl acetate): Hydrolysis yields acetic acid and isobutyl alcohol. Oxidation of isobutyl alcohol yields isobutyric acid, not acetic acid. (Incorrect) D. C2H5-COO-(CH2)2-CH3 (Propyl propanoate): This is CH3CH2COOCH2CH2CH3. Hydrolysis yields propanoic acid and propan-1-ol. Oxidation of propan-1-ol yields propanoic acid. All conditions are met. (Correct)
